Publications
Issue 5 of the AnNex Newsletter
Rich Gorman’s secondment to the RSPCA explored the social relations shaping the use of horseshoe crab blood within pharmaceutical endotoxin testing. This involved 13 stakeholder interviews, and has resulted in a stakeholder report alongside a peer-reviewed journal publication. Rich has also presented the research at industry conferences and academic seminars. This is the report and infographic produced for his research.
Animal research conducted outside of the laboratory faces various unique challenges, but has received only limited attention in terms of official guidelines, support, and statistics. To improve understanding, we held a workshop bringing together experts familiar with a variety of nonlaboratory animal research contexts (e.g., wildlife field sites, farms, fisheries, veterinary clinics, zoos). We collectively identified five key areas that we propose require further discussion and attention, which we present in this paper. While the workshop focused on research in the UK, our conclusions may have implications for similar work overseas.
Citizen science involves participation by members of the public in scientific research. In wildlife research, citizen scientists might be involved in the capture and handling of animals (e.g. via trapping, marking, and the use of tracking devices). Such work provides clear scientific benefits. However, it also comes with risks, including those to animal welfare. In this perspective piece we explore current regulations and questions around how best to regulate this work in order to ensure that it is undertaken in an ethical manner. We do this by drawing on qualitative social science research and stakeholder consultation with researchers, citizen scientists, and regulators in the UK.
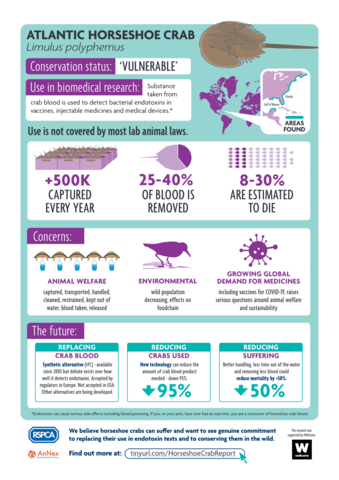
Endotoxin testing is a vital part of quality and safety control in pharmaceutical production. The primary method for this testing in North America and Europe is the limulus amebocyte lysate (LAL) test, a critical component of which is the blood of Atlantic horseshoe crabs (Limulus polyphemus). Procuring blood for LAL testing involves capturing and bleeding over 500,000 crabs from wild marine populations each year. Whilst efforts are made by manufacturers to return crabs to the sea following the collection of blood, there is a level of mortality and sub-lethal impact involved, prompting increasing discussions about welfare and ethics. The 3Rs – the ambition to where possible, replace, reduce, and refine the use of animals – are established and accepted worldwide as the best framework for governing animal-dependent science. However, the biomedical utilization of horseshoe crabs to produce the LAL test has rarely been viewed through a 3Rs framework. More recently, there has been a renewed attention on sustainable methods and alternatives to the LAL test. Drawing on in-depth qualitative interviews, this article examines stakeholder perspectives on opportunities for thinking with the 3Rs, considering current appetites to replace, refine, and reduce contemporary biomedical reliance on horseshoe crabs. The shape of conversations about the biomedical utilization of horseshoe crabs has shifted significantly in recent years, and the 3Rs are an important driver of change, offering the potential to advance the use of more sustainable methods, and realize the welfare considerations increasingly expected across science and society.
A good culture of care, empowering individuals within organisations to care and reflecting wider social expectations about care, is now a well-documented aspiration in managing practices of laboratory animal research and establishing priorities for patient and public health. However, there is little attention to how different institutional cultures of care interact and what happens to the accountabilities of caring roles and the entanglements of caring practices when institutional cultures meet.
This report explores what a social science perspective might add to understanding the debates surrounding the use of horseshoe crabs in endotoxin testing. It draws on qualitative research with stakeholders, alongside documentary and policy analysis to examine the various perspectives, positions, and sides of debates about horseshoe crabs. This report attempts to represent the wide diversity of stakeholder perspectives about the biomedical use of horseshoe crabs in a balanced manner.
Issue 4 is a COVID-19 special edition
Despite its increasing attention from animal welfare organisations, there is currently a lack of research which explores the numbers and species rehomed after being used in animal research. This paper, using a semi-structured questionnaire, explores rehoming practice across UK animal research facilities in order to provide an overall picture of current rehoming practice.
Environmental Ethics Statement