-------------------------------------------------------------------------------------------------------------------
Abstract
Event-related brain potentials (ERPs) were used to investigate the time course of memory processes following the presentation of faces. Following a phase in which subjects were asked to memorise faces presented on a computer screen (study phase) they had to distinguish the previously presented faces from others new to the experiment (test phase). We found that in a time period from 250-350 ms after onset of stimulus presentation ERPs show higher negativity for both repeated and novel faces in the test phase compared to the study phase. This situation dependent effect is most pronounced in occipito-temporal regions. We conclude that memory retrieval for faces is a sequential process: The early part of this process constitutes preparation for the retrieval of stored information, and a later part of the process comprises the discrimination between repeated and novel faces.
© 1998 Elsevier Science Ireland Ltd.
Keywords: Memory; Face-recognition; Event-related potentials; Study and recognition; Situation dependent effect
--------------------------------------------------------------------------------------------------------------------
The ability to recognise human faces is a prerequisite for social behaviour.
Studies on patients suffering from prosopagnosia (the inability to recognise
human faces) have revealed the existence of a mnemonic system exclusively
dealing with faces. Prosopagnosia has been described after lesions in the
occipito-temporal cortex of both hemispheres [3,4,9,13] or of only the
right hemisphere [5].
We chose event-related brain potentials (ERPs) as a tool to investigate
the time course of memory processes elicited by the presentation of faces
during both encoding and retrieval stages of processing. ERPs were
recorded from 15 healthy right-handed subjects (mean age 24.3 yrs, range
20-29 yrs, 8 female). Dexterity was assessed with a modified version of
the Edinburgh Inventory [10]. All subjects were paid and gave informed
consent.
Fig.1: Electrode positions on the scalp (circles) and their approximate
representation on the cortex (dots). Cz represents the position taken as
reference, PO9 and PO10 are taken as examples in Fig.2. Electrodes drawn
in grey represent sites where t-tests between the mean amplitudes of ERPs
in the interval from 250-350 ms after onset of stimulus presentation reveal
significant differences.A: Correctly classified repeated faces in the test
phase compared to their initial presentation in the study phase.B: The
same faces of the study phase compared to correctly classified novel faces
in the test phase.
Since the results of previous fMRI- and PET-studies [11,12] indicate that inferior temporal structures play a role in face-recognition, we placed some of the 38 scalp-electrodes more lateral and inferior to positions of the conventional 10/20-system (Fig. 1). The brain potentials were measured with Cz (vertex) as reference. Horizontal and vertical eye-movements were recorded using electrodes at the outer canthus of each eye as well as above and below the left eye. The potentials were amplified within the frequency band from DC to 100 Hz, sampled with 250 Hz and digitally filtered offline using a 70 Hz lowpass filter [7]. Eye blinks were also detected offline and their influence on EEG-data removed. All data were visually controlled for artefacts and affected trials were excluded.
Subjects had to memorise black and white photographs of young Caucasian adults without distinctive facial features, such as beards or spectacles, on a computer screen. The black and white photographs were carefully edited to maintain a standard brightness and contrast. The study phase comprised of 60 faces which were presented consecutively. After a rest period of two minutes the same 60 faces were presented again together with 60 new faces in a random order. The subjects had to classify the items as "old" or "new" by triggering one of two light barriers on each side of the right index finger. Five distinct study phases alternated with five distinct test phases.
The pictures were presented every 2500 ms for a period of 300 ms replacing a grey rectangle of the same size to reduce changes in luminance. The ERPs were averaged depending on the following three trial classes: correctly classified repeated faces in the test phase, their initial presentation in the study phase and correctly classified new faces in the test phase. The mean amplitudes of 12 time periods of 100 ms were calculated. The time periods overlapped for 50 ms and covered the time from stimulus onset until 650 ms after stimulus onset. The interval 500 ms before the stimulus onset was used as the baseline for ERP calculations. The resulting values of each recording site and interval were normalised [8], averaged and taken as dependent variables in a within-subject multivariate analysis of variance (Repeated Measures MANOVA) with RECORDING SITES and the TRIAL CLASSES as independent variables.
In the test phase subjects correctly identified 58% of the faces that were repeated from the study phase and 71% of the new faces were also correctly identified. In the light of the fact that the subjects were asked to remember 300 faces during the study phase, this clearly demonstrates the subject's concentration on our test and the activation of their memory.
ERPs following the presentation of correctly classified repeated faces
(test phase) were compared to the ERPs of their initial presentation (study
phase) and to those of correctly classified novel faces (test phase) (Fig.
2). Comparing all three trial classes, MANOVA yielded a topography-specific
ERP-effect of the experimental situation (interaction RECORDING SITES vs.
TRIAL CLASS) from 150-650 ms after onset of stimulus presentation (Greenhouse-Geisser
corrected; see Table 1). T-tests show that the ERPs following the presentation
of repeated and novel faces in the test phase were more negative-going
than ERPs following the presentation of faces in the study phase around
qthe same time.
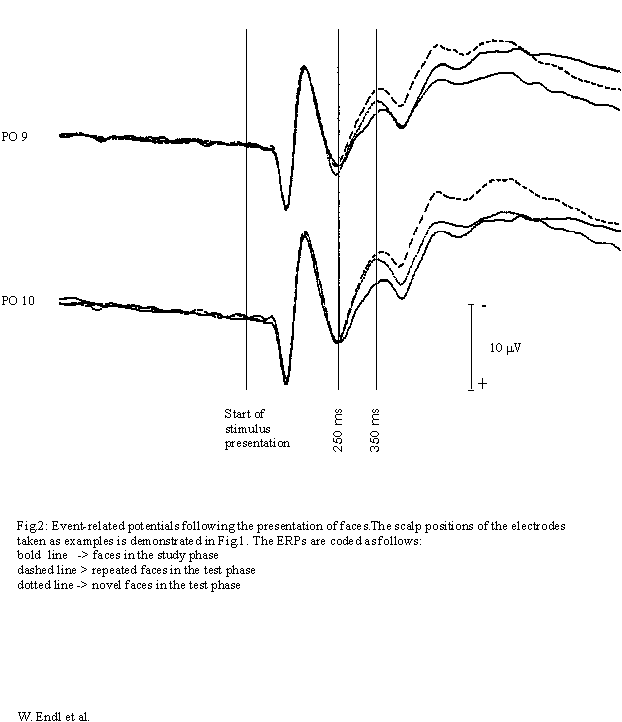
Table 1
Multivariate analysis of variance: p-values for the interaction between 'trial class' and 'recording site' (normalised data [8], Greenhouse-Geisser corrected).
|
Interval after stimulus onset (ms) |
0-100 |
50-150 |
100-200 |
150-250 |
200-300 |
250-350 |
300-400 |
350-450 |
400-500 |
450-550 |
500-600 |
550-650 |
|
study / repeat / novel |
.286 |
.478 |
.089 |
.001 |
.001 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
|
study / repeat |
.505 |
.480 |
.097 |
.002 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
|
study / novel |
.114 |
.340 |
.047 |
.000 |
.005 |
.001 |
.000 |
.000 |
.000 |
.000 |
.000 |
.000 |
|
repeat / novel |
.366 |
.616 |
.353 |
.325 |
.067 |
.019 |
.021 |
.060 |
.181 |
.337 |
.318 |
.316 |
At occipito-temporal recording sites ERPs following the presentation of faces are more negative-going in the test phase (repeated and novel) than in the study phase in the interval from 250 to 350 ms after onset of stimulus presentation. On the right hemisphere this effect is as pronounced for repeated as for novel faces. It is particularly significant at occipito-temporal recording sites (Fig. 1). Differences between ERPs following the initial presentation of faces and the presentation of novel faces in the test phase become only significant at occipito-temporal recording sites of the right hemisphere.
In the period from 250-350 ms after the onset of stimulus presentation the amplitude of the ERPs is significantly more negative-going following the recall of faces (test phase) compared to memorisation of faces (study phase). This situation dependent effect is most pronounced in occipito-temporal regions of the right hemisphere and precedes the so-called old/new effect which is the increase in amplitude due to the repeated presentation of a face compared to the presentation of a novel face in a test situation. It would seem that the mere effort to retrieve information about faces produces additional activity in regions which have been shown to deal with memory for faces in a variety of studies using scalp-recorded ERPs [2,6], intracortical recording of ERPs [1], fMRI [11] and PET [12]. This early situation dependent effect precedes the actual process of recognition (old/new effect) and can be regarded as an indication for the retrieval of stored information in the sequential process of face recognition. This early modulation of cortical activity has - to our knowledge - not been described before.
The authors would like to thank Prof. John Morgan and Dr. Michel-Ange Amorim for helpful comments on the manuscript. The project was supported by the Austrian Science Foundation.
[1] Allison, T., Ginter, H., McCarthy, G., Nobre, A., Puce, A., Luby, M. and Spencer, D., Face recognition in human extrastriate cortex, J. Neurophys., 71 (1994) 821-825.
[2] Begleiter, H., Porjesz, B. and Wang, W., A neurophysiologic correlate of visual short-term memory in humans, Electroencephalogr-Clin-Neurophysiol. 87 (1993) 46-53.
[3] De Renzi, E., Prosopagnosia in two patients with CT scan evidence of damage confined to the right hemisphere, Neuropsychologia 24 (1986) 385-389.
[4] De Renzi, E., Perani, D., Carlesimo, G.A., Silveri, M.C. and Fazio, F., Prosopagnosia can be associated with damage confined to the right hemisphere-an MRI and PET study and a review of the literature, Neuropsychologia 32 (1994) 893-902.
[5] Ettlin, T.M., Beckson, M., Benson, D.F., Langfitt, J.T., Amos, E.C. and Pineda, G.S., Prosopagnosia: a bihemispheric disorder, Cortex 28 (1992) 129-134.
[6] Hertz, S., Porjesz, B., Begleiter, H. and Chorlian, D., Event-related potentials to faces: the effects of priming and recognition, Electroencephalogr. Clin. Neurophysiol. 92 (1994) 342-351.
[7] Lindinger, G., Svasek, P., Lang, W. and Deecke, L., PC-supported 64-channel DC-EEG amplifier. In K.P. Adlassnig, G. Grabner, S. Bengtsson, R. Hansen (Eds.), Medical Informatics Europe 1991. Proceedings, Vienna, Austria, August 19-22. Lecture Notes in Medical Informatics 45. Springer, Berlin, Heidelberg, New York, 1991, pp.1005-1009.
[8] McCarthy, G. and Wood Ch. C., Scalp Distributions of Event-Related Potentials: An Ambiguity Associated with Analysis of Variance Models, Electroencephalogr. Clin. Neurophysiol. 62, (1985) 203 - 208.
[9] Michel, F., Poncet, M. and Signoret JL., Are the lesions responsible for prosopagnosia always bilateral?, Rev. Neurol. (Paris) 145 (1989) 764-770.
[10] Oldfield, R.C., The assessment and analysis of handedness: the Edinburgh inventory, Neuropsychologia 9 (1971) 97-113.
[11] Puce, A., Allison, T., Gore, J. and McCarthy, G., Face-sensitive regions in the human extrastriate cortex studied by functional MRI, J. Neurophys. 74 (1995) 1192-1199.
[12] Sergent, J., Ohta, S. and Macdonald, B., Functional neuroanatomy of face and object processing, Brain 115 (1992) 15-36.
[13] Takahashi, N., Kawamura, M., Hirayama, K., Shiota, J. and Isono, O., Prosopagnosia: a clinical and anatomical study of four patients, Cortex 31 (1995) 317-329.