G.NORTHOFF
Dept. of Psychiatry, University of Magdeburg, Germany
"This Ďnew orientationí, of which Jellife spoke, and of
which he himself was a notable exemplar, did not involve merely combining
neurological and psychiatric knowledge, but conjoining them, seeing them
as inseparable, seeing how psychiatric phenomena might emerge from the
physiological, or how, conversely, they might be transformed into it" (O.Sacks
1989, 157)
Address of Correspondence:
Georg Northoff, MD PHD
Associate Professor
Dept. of Psychiatry
University of Magdeburg
Leipziger Straße 44
39120 Magdeburg
Germany
Phone: 0049/(0)391-6714234
Fax: 0049/(0)391/6715223
E-mail: Georg.Northoff@medizin.Uni-Magdeburg.de
SUMMARY
Differentialdiagnosis of motor symptoms, as for example akinesia, may be difficult since they may be either of neurologic, as for example Parkinsonís, or psychiatric, as for example catatonia, origin leading to a so-called "conflict of paradigms". Despite different origins such symptoms may clinically be more or less similar which may reflect functional brain organisation in general and cortical-subcortical relations in particular. It is therefore hypothesized that similarities and differences between Parkinsonís as a motor disorder and catatonia as a psychomotor disorder may be accounted for by functional differences between "top-down modulation" and "bottom-up modulation" between prefrontal/frontal cortex and basal ganglia implying double dissociation between both diseases with regard to underlying pathophysiology.
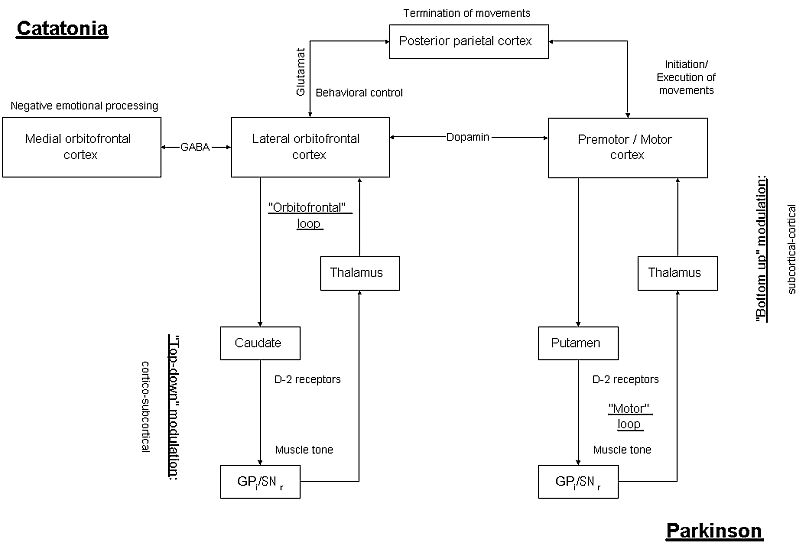
Catatonia can be characterized by concomittant motor, emotional, and behavioral symptoms which may be accounted for by dysfunction in orbitofrontal-prefrontal/parietal cortical connectivity as a form of "horizontal i.e. cortico-cortical modulation". Furthermore alteration in "top-down modulation" of caudate and other basal ganglia by gaba-ergic mediated orbitofrontal cortical deficits may account for motor symptoms in catatonia. Parkinsonís in contrast can be characterized by predominant motor symptoms which may be accounted for by altered "bottom-up modulation" between dopaminergic mediated deficits in striatum and premotor/motor cortex. Due to connectional asymmetry i.e. unidirectionality in prefronto-premotor/motor cortical connections, there is no further dysregulation in other prefrontal cortical areas in Parkinsonís as it is reflected in absence of major psychiatric symptoms in such patients.
It is concluded that comparison between Parkinsonís and catatonia may reveal the nature of both "top-down modulation" and "bottom-up modulation" in further detail. Furthermore difference between Parkinsonís as a motor and catatonia as a psychomotor disorder may be accounted for by pecularities in "horizontal i.e. cortico-cortical modulation" which, unlike "top-down and bottom-up modulation" as forms of "vertical modulation", may be unidirectional and thus asymmetric not allowing for direct modulation of prefrontal cortical areas by premotor/motor cortex.
Key-words: Catatonia - Parkinsonís - Top-down modulation
- Bottom-up modulation - Horizontal modulation
Differentialdiagnosis of motor symptoms, as for example akinesia, may be difficult since they may be either of neurologic, as for example Parkinsonís, or psychiatric, as for example catatonia, origin leading to a so-called "conflict of paradigms". Despite different origins such symptoms may clinically be more or less similar which may reflect functional brain organisation in general and cortical-subcortical relations in particular. It is therefore hypothesized that similarities and differences between Parkinsonís as a motor disorder and catatonia as a psychomotor disorder may be accounted for by functional differences between "top-down modulation" and "bottom-up modulation" between prefrontal/frontal cortex and basal ganglia implying double dissociation between both diseases with regard to underlying pathophysiology.
It is concluded that comparison between Parkinsonís and
catatonia may reveal the nature of both "top-down modulation" and "bottom-up
modulation" in further detail. Furthermore difference between Parkinsonís
as a motor and catatonia as a psychomotor disorder may be accounted for
by pecularities in "horizontal i.e. cortico-cortical modulation" which,
unlike "top-down and bottom-up modulation" as forms of "vertical modulation",
may be unidirectional and thus asymmetric not allowing for direct modulation
of prefrontal cortical areas by premotor/motor cortex.
1. INTRODUCTION
Clinical diagnosis and therapy of neuropsychiatric disturbances is often made rather difficult by similarities between symptoms caused by different disturbances either neurologic or psychiatric. The symptom of akinesia can be considered as a typical example of such differentialdiagnostic problems since it may be caused either by Parkinsonís, classified as a neurological disease, or catatonia, usually classified as a psychiatric disease. In addition the same symptom i.e. akinesia may be accompanied by different psychological alterations either depression, as in Parkinsonís, or uncontrollable anxieties, as in catatonia. Consequently consideration of both symptomatic origin and complexity makes classification of diseases as either neurologic or psychiatric rather difficult which is reflected in a so-called "conflict of paradigms" pointing out the inability to draw a clear dividing line between neurologic and psychiatric disturbances.
If symptoms of different origin i.e. psychiatric or neurologic look more or less similar one has to assume similar or at least overlapping pathophysiological substrates accounting for both similarities and differences in symptomatology between both kinds of disturbances. As such pathophysiological mechanisms may reflect functional brain organisation allowing for such similarities in symptoms of different origin. With regard to Parkinsonís and catatonia functional relation between cortical areas i.e. prefrontal/frontal cortex and subcortical structures i.e. basal ganglia may account for similarity in motor symptoms. Functionally relation between prefrontal/frontal cortex and basal ganglia can be characterized by various "functional circuits" including "orbitofrontal and motor loop" (see Mastermann and Cummings 1997 for a nice overview) allowing for both "top-down and bottom-up modulation". Consequently if one wants to account for similarity in symptoms of different origin, as it seems to be the case in Parkinsonís and catatonia, one has to investigate these "functional circuits" which, in addition, may enhance our understanding of "top-down modulation" in general. Comparison between pathophysiological mechanisms underlying Parkinsonís and those subserving catatonia may reveal the nature of cortical-subcortical relationship and thus of "vertical modulation" i.e. "top-down and bottom-up modulation" in further detail. Thereby the following hypothesis are postulated serving as the thread of the concept to be developped: (i) apparent similarity with underlying differences in motor symptoms between Parkinsonís and catatonia; (ii) differences in psychiatric i.e. affective and behavioral symptoms between Parkinsonís and catatonia; (iii) "double dissociation" between catatonia and Parkinsonís with regard to underlying pathophysiological mechanisms accounting for differences between Parkinsonís and catatonia; (iv) opposite kinds of "vertical modulation" between prefrontal/frontal cortex and basal ganglia in Parkinsonís, as characterized by "bottom-up modulation", and catatonia, as characterized by "top-down modulation" accounting for apparent similarity and underlying differences in motor symptoms; (v) presence/absence of alterations in cortico-cortical connectivity as a kind of "horizontal modulation" in catatonia and Parkinsonís respectively accounting for difference between both with regard to psychiatric symptoms.
In a first step we describe similarities and differences
in clinical symptoms and therapy between Parkinsonís and catatonia which
is followed by illustration of neuropsychological and pathophysiological
findings in second step. In a third step we develop pathophysiological
hypothesis for the different kinds of symptoms observed in Parkinsonís
and catatonia. Finally we infer conclusions with regard to "top-down and
bottom-up modulation" as kinds of "vertical modulation" and cortico-cortical
modulation as kinds of "horizontal modulation".
2. CATATONIA AS A PSYCHOMOTOR SYNDROME: COMPARISON WITH PARKINSONíS AS A MOTOR SYNDROME
2.1. Motor Symptoms
Catatonia is a rather rare (incidence: 2-8% of all acute admissions) psychomotor syndrome which can be associated with psychiatric disturbances such as schizophrenia (one subtype is denoted as "catatonic schizophrenia") and manic-depressive illness as well as with various neurological and medical diseases (Gelenberg 1976, Taylor 1990, Northoff 1997). Some authors (see Northoff 1997 for an overview) consider periodic catatonia as an idiopathic disease showing psychomotor characteristics of catatonic syndrome while not beeing associated with any other kind of disease. Parkinsonís is a motor syndrome which can be either of idiopathic i.e. primary or symptomatic i.e. secondary nature. In the first case one speaks of Parkinsonís disease, which may be considered as a nosological analogue of periodic catatonia, whereas in the second case one generally speaks of Parkinsonís syndrome which, similar to catatonia, may be associated with various neurological and medical diseases.
The most characteristic feature of catatonia is posturing where patients show a specific, uncomfortable, and often bizarre position of parts of their body against gravity with complete akinesia in which they remain for hours, days, and weeks (and in earlier times even for years; see Figure 1). If that position is taken actively and internally by the patient himself one speaks of 'posturing', if such a position can be induced passively and externally by the examiner one speaks of 'catalepsy'. Posturing can occur in limbs ("classic posturing"), head ("psychic pillow"), and eyes ("staring").
We saw one patient who postured every morning during shaving. He started to shave himself and remained then, with the razor in his hand and a lifted arm, for hours in that position until his wife came in and "depositioned" him. Another example is that of a woman who, every morning by opening her wardrobe, remained in a position with a lifted arm keeping the door of the wardrobe in her hand. Both patients were admitted into clinic where they did neither speak nor move at all. On admission it was possible to "position" their limbs in the most bizarre and uncomfortable positions against gravity without any resistance by the patients themselves. Once the examiner positioned the limbs into one particular position they remained in that position without showing even the slightest change.
These cases are typical examples of posturing and catalepsy where patients are well able to initate and execute movements but seem to be unable to return to the initial or resting position in order to start a new movement. Similar to Parkinson's, catatonic patients do show akinesia but, unlike parkinsonian patients, only in association with posturing and catalepsy. Furthermore, in contrast to Parkinson's, catatonic akinesia is not necessarily accompanied by muscular hypertonus i.e. rigidity in catatonia since patients may also show muscular normo- or hypotonus (Northoff 1997). Even if catatonic patients show muscular hypertonus it is not cogwheel rigidity typical for parkinsonís but rather a smooth type of rigidity which is called flexibilitas cerea (Northoff et al. 1999). In addition to hypokinetic features catatonic patients may show intermittent and fluctuating hyperkinesias like stereotypies, dyskinesias, and tics which, unlike in Parkinsonís, are independent from medication.
Catatonic patients are well able to "plan", "initiate", and "execute" movements which could be demonstrated in ball-experiments. We performed systematic ball-experiments in 32 catatonic patients in an acute akinetic state before they received any medication (i.e. lorazepam) (see Northoff et al. 1995). To our surprise almost all patients, despite showing concomittant akinesia and posturing, were able to play ball either with the hands or with the legs. Patients were able to catch and throw the ball, being slightly better during external intiation (i.e. catching) than internal initiation (i.e. throwing). Most patients however remained in a final posture keeping the ball in a position against gravity being apparently unable to change posture and terminate the respective movement. Subjectively catatonic patients experienced these ball-experiments as ""funny and relaxing" and as "taking off my inner tension" while they were not aware of their inability to terminate movements i.e. posturing. Furthermore, in contrast to Parkinsonís, posturing in catatonic patients can not be reversed by external sensory stimulation, as for example, drawing a line in front of the feet. Accordingly catatonic patients did not experience any starting problems or deficits in "internal initiation".
In summary catatonia and Parkinsonís can be characterized
by clinical similarities, as it is reflected in akinesia and rigiditiy,
and differences, as it is reflected in posturing/initiation and cogwheel
rigidity/flexibilitas cerea, with regard to motor symptoms.
2.2. Behavioral and affective symptoms
In addition to motor symptoms, catatonia can be characterized by concomittant behavioral and affective anomalies. Behavioral anomalies include mutism (patients do not speak anymore at it was the case in both patients described above), stupor (no reaction to the environment), automatic obedience (patients do everything what they are asked for), negativism (patients do always the opposite of what they are asked for), echolalia/praxia (patients do repeat sentences or actions from external persons several times or even endless), perseverative-compulsive behavior (uncontrollable repetetive behavioral patterns) and mitmachen/mitgehen (patients do always follow other persons and make the same as they do). In contrast to catatonia such behavioral anomalies cannot be observed in Parkinsonís which can be characterized predominantly by motor symptoms.
Affective alterations in catatonia include strong anxieties or euphoria/happiness, staring, grimacing, and inadaequate emotional reactions. Catatonic patients may show compulsive emotions (involuntary and uncontrollable repetetive emotional reactions), emotional lability (labile and unstable emotional reactions), agression (often accompanied by extreme emotional states such as anxiety or rage), excitement (extreme hyperactivity with extreme and uncontrollable emotional reactions), affective latence (long time to show emotional reactions), ambivalence (simultaneous presence of conflicting emotions), and flat affect (decreased and/or passive emotional reactivity) - symptoms which as such are not present in parkinsonian patients. Parkinsonís may be characterized by depression whereas they do neither show such an uncontrollable intensity of emotions nor a comparable variety of emotional reactivity as catatonic patients.
In summary catatonia can be characterized by strong affective
and bizarre behavioral anomalies which as such do not ocurr in Parkinsonís.
2.3. Therapy
Therapeutically 60-80% of all acute catatonic patients react to lorazepam, a GABA-A receptor potentiator, either almost immediately within the first 5-10 minutes or within 24 hours (Rosebush et al. 1990, Northoff et al. 1995, Bush et al. 1996) whereas chronic catatonic patients show no improvements on lorazepam (Ungvari et al. 1999). If lorazepam does not work some catatonic patients show gradual and delayed improvements (within 2 to 4 days) on the NMDA-antagonist amantadine (Northoff et al. 1997, 1999) and/or on electroconvulsive treatment (ECT) (Fink et al. 1993, Petrides et al. 1997).
Parkinsonian patients can be therapied primarily with dopaminergic substances i.e. L-Dopa and D1/2 receptors agonists whereas, unlike in catatonia, lorazepam and other benzodiazepines remain therapeutically ineffective. Similar to catatonia Parkinsonís may be therapeutically relieved by the NMDA-antagonist amantadine (Merello et al. 1999). In addition to pharmacotherapy surgical therapies with implantation of either electrodes or fetal tissue in specific structures of the basal ganglia (putamen, caudate, subthalamic nuclei, internal pallidum) may be applied especially in drug-resistant patients.
In summary treatment in catatonia and Parkinsonís can
be characterized by differences (Gaba-ergic agents versus dopaminergic
agents) and similarities (NMDA-antagonists).
2.4. Subjective experience
In order to further reveal the nature of psychological alterations and their relation to motor symptoms we investigated subjective experience in catatonic patients (which, due to mutism and stupor, is possible only retrospectively) with a self-questionnaire and compared them with akinetic parkinsonic patients and non-catatonic depressive and schizophrenic patients (see Northoff et al. 1998 for details).
Parkinsonian patients severely suffered from akinesia, they felt "locked into my body", and "wanted to move but was unable to do so". Catatonic patients, in contrast, did not realize "any alterations in my movements" and said that "they (the movements) were completely normal". Asked why they positioned their limbs in a particular posture they either answered "There was nothing abnormal with my movements" or couldn't say anything. The patient posturing during shaving said "My movements were completely normal and I could shave in the normal way without any time delay". No patient said that he subjectively suffered from any changes in his movements. Moreover no catatonic patient reported any feeling of pain or tiredness even if he postured and remained in the same position for hours (n=5), days (n=10) or weeks (n=5). Instead of changes in their movements many catatonic patients reported extremely intense emotions which they experienced as "uncontrollable". Patients "felt totally blocked" by these emotions which "overwhelmed me" and "lead to a blockade of my self". The dominating emotion was anxiety (due to paranoid delusions, acoustic hallucinations, depressive mood or traumatic experiences). For example, the shaving patient presented above, said that "I couldn't control my emotions anymore, they were overflooding me so that I had the feeling that I was just anxiety". Nevertheless some patients reported rather positive emotions like euphoria which, however, similar to anxiety, they were unable to control anymore. One patient, for example, became catatonic every time (5 times in total) when she fall in love reporting the following: "I am so happy when I fell in love, this feeling really overwhelms me so that I can't control it anymore. Every time when I fell in love I am admitted to clinic I don't understand this".
Catatonic patients did not subjectively experience any "sensation of effort" during posturing. Although they kept their limbs or head in a position against gravity, where every normal person and patient with Parkinson's would feel a "sensation of tiredness or pain", catatonic patients do not experience any "tiredness", pain, or a "sensation of effort" during posturing. For example, catatonic patients lying in the bed may keep up their head for hours or even days (i.e. a so-called ,psychic pillowĎ) without getting tired and/or reporting any feeling of tiredness. Asking these patients with such a "psychic pillow" they answer "My head was in a completely normal position, I wasn't tired at all"; instead they rather seem to experience a sense of weightlessness.
No catatonic patient was able to give an account of the position in which he kept his limbs thus remaining unaware of posturing. It seems as if they have no access to any kind of subjective experience of the actual spatial position during posturing Ė the "objective position" and the corresponding "subjective experience" of the spatial position seem to be decoupled from each other. Furthermore they are not aware of the "consequences of their movements" (Snowdon et al. 1998): The patient posturing during shaving claimed that he finished shaving every morning completely without any time delay so that he wasnít aware of the "consequences of posturing". Finally catatonic patients do neither show any objective nor any kind of subjective sensory abnormality so that alterations in subjective experience cannot be accounted for by sensory dysfunction.
Almost all catatonic patients reporting strong, intense, and uncontrollable emotions responded well to lorazepam whereas patients without such emotional experiences did not respond well to lorazepam. Non-responders to lorazepam, as for example the above described patient posturing in front of her wardrobe, rather experienced a "blockade of my will with contradictory and ambivalent thoughts about my dresses so that I couldn't decide myself". For several days this patient stood in front of her wardrobe remaining in the same quite uncomfortable position with raised arms on the tip of her toes. She wasn't aware of any alterations in her movements denying any feeling of tiredness during that position ("I wasn't tired at all"). The present hypothesis primarily focuses on catatonic responders to lorazepam. This is important to mention since responders and non-responders may be characterized by distinct underlying pathophysiological mechanisms (Northoff et al. 1995, 1998, Ungvari et al. 1999). All catatonic patients experienced their admission on a psychiatric ward as terrible ("I thought it was the hell") and/or could not understand it ("I was so happy, there was no reason for admission this time"). Moreover they very well remembered the physician and other persons who treated them on admission. Consequently catatonic patients seem to show no deficits in memory (except in working memory; see below).
In summary subjective experience differs between catatonic
and parkinsonian patients with regard to motor symptoms (motor anosognosia
versus motor awareness) and psychological state (anxiety versus depressive
reaction).
3. NEUROPSYCHOLOGICAL AND PATHOPHYSIOLOGICAL FINDINGS IN CATATONIA AND PARKINSONíS
3.1. Neuropsychological findings
We pointed out that the ability to registrate the spatial position of movements, as required for "Termination of movements" (see above), does involve spatial abilities which may be related to right posterior parietal cortical function. We therefore investigated postacute akinetic catatonic patients with neuropsychological tests for measurement of spatial abilities (Northoff et al. 1999). Among other measures we applied the Visual-Object-Space and Perception Test (i.e. VOSP) a test specifically designed for measurement of spatial abilities related to right parietal cortical function.
Catatonic patients showed significantly lower performance in VOSP compared to psychiatric and healthy controls (Northoff et al. 1999). The specifity of this finding is further underlined by the fact that catatonic patients differed from psychiatric controls neither in any other visuo-spatial test unrelated to right parietal cortical function nor in any other neuropsychological measure such as general intelligence, attention, and executive functions. Furthermore catatonic patients showed significant correlations between right parietal cortical visuo-spatial abilities (as measured with VOSP) and attentional abilities (as measured with d2 and CWI) which were neither present in psychiatric controls nor in healthy subjects (Northoff et al. 1999). In addition motor symptoms in catatonia correlated significantly with both visuo-spatial abilities and attentional function.
Catatonia may be characterized by relatively intact psychological functions concerning attention, executive functions, general intelligence, and non-right parietal visuo-spatial abilities whereas spatial abilities specifically related to right parietal cortex may be altered in catatonic patients distinguishing them from non-catatonic psychiatric controls. In addition catatonic patients show severe deficits in neuropsychology testing for orbitofrontal cortical function (unpublished observations) as it is reflected in the gamble test or game for chance test designed by Bechara et al. (1997). Therefore one may hypothesize that catatonic patients are neither able to decide in an emotionally-guided intuitive way nor to perform on-line monitoring as required for decisions.
Parkinsonian patients in contrast do show severe neuropsychological deficits in executive functions (Wisconsin Card Sorting test, Verbal fluency, etc.) which among others include abilities of categorization, shifting, sequencing etc. as subserved by dorsolateral prefrontal cortical function. In contrast to catatonia Parkinsonís can neither be characterized by deficits in visuo-spatial attention specifically related to right parietal cortical function nor by alterations in the gamble test specifically designed for orbitofrontal cortical function.
In summary catatonia can be characterized by specific
deficits in visuo-spatial attention, as related to right parietal cortical
function, and emotionally-guided intuitive decisions, as related to orbitofrontal
cortical function. Parkinsonís in contrast can be characterized by specific
alterations in executive functions as predominantly related to lateral
prefrontal cortical function.
3.2. Postmortem findings
Early postmortem studies in the preneuroleptic area revealed discrete but not substantial alterations in the basal ganglia (Caudate, N. accumbens, Pallidum) and thalamus (see Bogerts et al. 1985 and Northoff 1997 for an overview). Since these early studies yielded rather inconsistent results, they were never pursued. These findings were made in patients with catatonic schizophrenia so it remains unclear whether these alterations are specifically related to either catatonia itself or schizophrenia. In contrast neuropathologic investigations of catatonic syndrome rather than on catatonic schizophrenia are currently not available. Most studies were performed on brains of patients who never were exposed to neuroleptics so that these alterations in basal ganglia cannot be related to neuroleptic (antipsychotic) modulation. Nevertheless findings should be considered rather cautiously since the methods and techniques available at that time may produce artifacts by themselves.
In contrast to catatonia substantial alterations in postmortem brains can be found in Parkinsonís. Parkinsonís disease as primary Parkinsonís can be characterized by degeneration of dopaminergic cells in substantia nigra pars compacta leading consecutively to degeneration in striatum especially putamen and caudate. In many cases of secondary Parkinsonís vascular or other kinds of alterations may be observed in striatum.
In summary valid postmortem results in catatonia are not
available at present, the older ones as characterized by rather insufficient
methods showing discrete alterations in basal ganglia. In contrast Parkinsonís
can be characterized by major degeneration of dopaminergic cells in substantia
nigra and its pathway to striatum.
3.3. Animal models
DeJong (1930) performed various experiments with the D2-receptor antagonist bulbocapnine which induced catatonia in animals with neocortex (mice, rats, cats) whereas in animals without neocortex catatonia could not be induced. Lower (1-2mg) doses of bulbocapnine leaded to catalepsy whereas higher doses (4-5mg) induced impulsive and convulsive reactions. As demonstrated by Loizzo et al. (1971) amantadine as an NMDA-antagonist lead to reversal of bulbocapnine-induced catatonia. However relying on own experiments, which remained unpublished, bulbocapnine-induced catatonia very much resembled haloperidol-induced catalepsy and, similar to the latter, the former could not be resoluted by lorazepam, as it is the case in human catatonia (see above). Such an assumption is further supported by findings of an inhibitory effect of bulbocapnine on dopamine synthesis (Shin et al. 1998). Consequently one cannot be sure whether DeJong really describes catatonia or rather catalepsy as similar to neuroleptic-induced catalepsy.
Stille and Sayers (1975) induced a catatonic-like reaction in animals with strong sensory stimuli (electric footshock) and postulated an excitement of the ascending arousal system i.e. formatio reticularis with overexcitation of the striatal system via thalamic nuclei. Injection of the GABA-A antagonist bicucullin into dopaminergic cells of the ventral tegmental area (VTA) induced a catatonic-like picture in cats with increased arousal, withdrawal, anxiety, staring, and catalepsy (Stevens 1974) which can be observed also after injection of morphine so that one may even speak of a "morphine-induced catatonia". At present no further investigation of animal models of catatonia are known to me.
Animals models of Parkinsonís focus on specific lesion of nigrostriatal dopaminergic cells and pathways as provided by 6-OHDH and MPTP models.
In summary there are no consistent animal models of human
catatonia at present. Models focusing on dopaminergic modulation may be
problematic and may rather reflect neuroleptic-induced catalepsy than human
catatonia. Rather modulation of gaba-ergic system or/and induction of stress
may lead to pictures resembling human catatonia. In contrast animal models
of Parkinsonís may be characterized by specific modulation of dopaminergic
system in either substantia nigra or striatum.
3.4. Structural imaging
A computerized tomographic (Head CT) investigation of 37 patients with catatonic schizophrenia showed a diffuse and significant enlargement in almost cortical areas, especially in the left fronto-parietal area which, in addition, correlated significantly with illness duration (see Northoff et al. 1999). Alterations in temporal cortical areas were present in all three subtypes of schizophrenia whereas catatonic schizophrenia could be specifically characterized by prefrontal and parietal enlargement. Other authors (Joseph et al. 1985, Wilcox 1991) observed a cerebellar atrophy in catatonic patients which however was neither investigated systematically nor quantitatively. To my knowledge no study specifically investigating catatonic syndrome (and not only catatonic schizophrenia as a subtype) has been published so far.
In summary findings in structural imaging in catatonia
suggest cortical involvement predominantly in prefrontal and parietal cortex
whereas in Parkinsonís subcortical structures i.e. the basal ganglia are
altered.
3.5. Functional imaging
3.5.1. Regional cerebral blood flow
Investigations of regional cerebral blood flow (rCBF) in catatonia showed a right-left asymmetry in basal ganglia with hyperperfusion of the left side in one patient (Luchins 1989), a hypoperfusion in left medial temporal structures in two patients (Ebert et al. 1992), an alteration in right parietal and caudal perfusion in one patient (Liddle 1994), a decreased perfusion in right parietal cortex in six patients with catatonic schizophrenia (Satoh et al. 1993), and a decreased perfusion in parietal cortex with improvement after ECT in one patient (Galynker et al. 1997). Importance of right parietal cortex in catatonia is further underlined by observation of posturing in patients with isolated lesions in right parietal cortex (Fukutake et al. 1993, Saver et al. 1993).
A systematic investigation of rCBF in SPECT in 10 postacute catatonic patients showed decreased perfusion in right posterior parietal and right inferior lateral prefrontal cortex compared to non-catatonic psychiatric and healthy controls. In addition, decreased perfusion in right parietal cortex correlated significantly with motor and affective symptoms as well as abnormally with visual-spatial and attentional neuropsychological abilities (Northoff et al. 2000).
In psychiatric and healthy controls VOSP correlated significantly with right lower parietal and right lower lateral prefrontal cortical r-CBF and Iomazenil binding (reflecting the function of GABA-A receptors) whereas in catatonia none of these correlations were found (Northoff et al. 1999). In addition r-CBF was significantly decreased in both areas right posterior inferior parietal cortex and right lower lateral prefrontal cortex. Catatonic motor symptoms correlated significantly with VOSP, right lower parietal r-CBF and iomazenil binding, measuring GABA-A receptor function indirectly, in right lower lateral prefrontal cortex (Northoff et al. 1999).
Parkinsonís can be characterized by deficits of r-CBF in SMA, motor cortex, and caudate whereas no major alterations in prefrontal and parietal cortex can be observed (see Jahanshahi and Frith 1998).
In summary investigation of regional cerebral blood flow
shows deficits in right lower inferior prefrontal and right parietal cortex
in catatonia whereas Parkinsonís may be characterized by r-CBF deficits
in motor cortex, SMA, and basal ganglia.
3.5.2. Motor activation
Functional imaging performed during motor activation (i.e. sequential finger opposition) showed reduced activation of the contralateral motor cortex (i.e. MC) in right hand performance, ipsilateral activation was similar for both patients and (medication-matched) controls. There were no differences in activation of the supplementary motor area (i.e. SMA). During left hand performance right-handed patients showed more activation in ipsilateral motor cortex, a reversal from the normal pattern of activation in which the contralateral side shows four to five times more activation than the ipsilateral side.
During motor activation parkinsonian patients show major deficits predominantly in SMA, which receives most afferences from thalamic (motor) nuclei, to a lesser degree in MC, which receives not as many afferences from thalamic (motor) nuclei), and off course in basal ganglia, i.e. in striatum. In contrast to catatonia no alteration in laterality during motor performance can be observed in Parkinsonís (Jahanshahi and Frith 1998).
In summary catatonia may be characterized by alterations
in laterality in motor cortex during motor performance SMA activation remaining
intact. Parkinsonís in contrast show major deficits in activation of SMA
and to a lesser degree in motor cortex the latter showing no alterations
in laterality.
3.5.3. Emotional-motor activation
Based on subjective experiences showing intense emotional-motor interactions, an activation paradigm for affective-motor interaction was developed and investigated in fMRI and MEG (magnetoencephalography) in catatonic patients comparing them with non-catatonic psychiatric and healthy controls (Northoff et al. 2000). During negative emotional stimulation catatonic patients showed a specific deficit in orbitofrontal cortical activation with a shift to anterior cingulate and medial prefrontal cortex which, in addition, was related with abnormal orbitofrontal-premotor/motor connectivity (Northoff et al. 2000).
Catatonic patients showed alterations in right orbitofrontal cortical activation in both FMRI and MEG, which instead shifted to and could therefore be localized in anterior cingulate/medial prefrontal cortex, during negative emotional stimulation. Behavioral and affective catatonic symptoms correlated significantly with reduced orbitofrontal cortical activity whereas motor symptoms correlated with premotor/motor activity. In addition decreased activity in right medial and lateral orbitofrontal cortex during negative emotional stimulation leaded to abnormal functional connectivity between orbitofrontal and premotor/motor cortex in catatonic patients (Kötter and Northoff 2000).
Parkinsonís in contrast can be characterized by altered activation in left dorsolateral prefrontal cortex and anterior cingulate during emotional stimulation whereas orbitofrontal cortical function remained unaffected. (see Mayberg et al. 1999).
In summary catatonia can be characterized by a deficit
in predominantly right orbitofrontal cortical activation and abnormal orbitofrontal-premotor/motor
connectivity during negative emotional stimulation whereas Parkinsonís
does show alterations only in left dorsolateral prefrontal cortex and anterior
cingulate but not in orbitofrontal cortex.
3.5.4. On-line monitoring
Based on phenomenological observation of posturing (see above) we investigated the ability of on-line monitoring as an essential component of working memory in catatonia since the inability to terminate movements may be related to a deficit in on-line monitoring of the spatial position of movements.
Since both on-line monitoring and active storage/retrieval can be considered as parts of working memory (Petrides 1995, Leary et al. 1999) we investigated working memory with an one-back and two-back task in catatonic patients in FMRI (see Leschinger et al. 2000).
Catatonic patients showed significantly worse performance in both one-back and two-back tasks such that their deficit seems not to be limited to active storage/retrieval. In the latter case one would have expected worse performance in the two-back task only. Instaed catatonia can be characerized by concomittant problems in on-line processing and monitoring accounting for bad performance in the one-back task. Catatonic patients showed significantly decreased activation in right lateral orbitofrontal including ventrolateral prefrontal cortex (i.e. VLPFC) during the working memory task in FMRI compared to psychiatric and healthy controls (Leschinger et al. 2000). In addition catatonic behavioral symptoms correlated significantly with activation in right lateral orbitofrontal cortex whereas motor symptoms showed a significant relationship with right dorsolateral prefrontal activity.
Investigation of working memory in Parkinsonís did show alteration in lateral prefrontal cortex especially in left dorso-lateral prefrontal cortex (i.e. DLPFC) whereas orbitofrontal cortical function including the ventrolateral prefrontal cortex remained intact (Jahanshahi and Frith 1998).
In summary catatonia can be characterized by major deficits
in on-line monitoring and right lateral orbitofrontal i.e. ventrolateral
prefrontal cortical (VLPFC) function whereas parkinsonian patients do rather
show deficits in left dorso-lateral prefrontal cortical (i.e. DLPFC) function.
3.6. Electrophysiological findings
3.6.1. Initiation in catatonia and Parkinsonís
Generation of movements can be characterized by "Plan/Strategy", "Initiation", and "Execution" which can be investigated by movement-related cortical potentials (i.e. MRCP) which we therefore investigated in catatonic patients comparing them with non-psychiatric and healthy subjects (see Northoff et al. 2000).
We investigated MRCP's during finger tapping in 10 postacute akinetic catatonic patients, 10 non-catatonic psychiatric controls (same underlying diagnosis, same medication, same age and sex), and 20 healthy controls (Northoff et al. 2000). We found neither significant differences in amplitudes between catatonic and non-catatonic subjects in early MRCP's; i.e. in early readiness potential (early RP) reflecting "Plan/Strategy" and "Initiation" of movements in DLPFC and anterior SMA; nor in amplitudes in late MRCP's i.e. in late readiness potential (late RP) and movement potential (MP) reflecting "Execution" of movements in posterior SMA and motor cortex.
Parkinsonian patients do show reduction of amplitude in early and late MRCPís which can be modulated by dopaminergic agents resulting in an increase of amplitude (Dick et al. 1987, 1989, Jahanshahi et al. 1995, Jahanshahi and Frith 1998).
In summary catatonia can be characterized by intact early
and late readiness potentials reflecting the preserved ability of "Plan/Strategy",
"Initiation", and "Execution" of movements in such patients. In contrast
parkinsonian patients do show severe deficits in "Initiation" and "Execution"
as it is electrophysiologically reflected in alterations in early and late
readiness potentials.
3.6.2. Termination in healthy subjects
Phenomena like posturing and catalepsy occuring in patients with right parietal cortical lesions (without showing any deficits in "Initiation" and "Execution") (Saver et al. 1993, Fukutake et al. 1993) (see above) suggest that visuo-spatial attention and right parietal cortical function may be necessary for on-line monitoring of the spatial position for movements and thus for termination of the latter. In a first step we therefore investigated termination of movements in healthy subjects with electrophysiological measurements of movement-related cortical potentials (MRCP) (Northoff et al. 2000 , Pfennig et al. 2000).
We compared ,normal' MRCP (i.e. MRCP) as obtained by finger tapping with MRCP for simple lifting so that the finger had to be kept up without going back into the intial position (MRCP 1) reflecting "Plan"/"Strategy", "Initiation", and "Execution" of finger tapping with exclusion of "Termination". "Termination" of movements was measured by the lowering of the finger after some seconds of posturing (MRCP 2) reflecting "initiation of termination" and "execution of termination" (see below). MRCP 1 and 2 differed significantly in various onsets and amplitudes from MRCP so that neither MRCP 1 nor MRCP 2 can be equated with MRCP for simple finger tapping. In addition, we obtained significant differences between MRCP 1 and MRCP 2 the latter showing significantly lower amplitudes in early parietal MRCPís, earlier onset of movement potential, and more posterior parietal localization of underlying dipoles than the former (Northoff et al. 2000, Pfennig et al. 2000).
Lorazepam as a GABA-A potentiator had a differential influence on early and late components of MRCPís during Initiation and Termination. During Initiation lorazepam lead to a delay in onsets of late MRCPís in frontal electrodes (MRCP 1) whereas during Termination (MRCP 2) early onsets in parietal electrodes were delayed. These results were further underlined by dipole source analysis. MRCP 1 reflecting "Plan"/"Strategy", "Initiation", and "Execution" showed dipole sources in anterior/posterior SMA and motor cortex whereas in MRCP 2 reflecting "Termination" the early dipole was located initially in right posterior parietal cortex shifting to posterior SMA and motor cortex.
The following conclusions with regard to "Termination" of movements can be drawn. First some kind of initiation must be involved since otherwise there would have been no readiness potential Ė we call this the "initiation of termination". Second the "initiation for execution" (i.e.MRCP 1) and the "initiation for termination" (i.e. MRCP 2) can apparently be distinguished from each other since otherwise there would have been no differences in amplitudes in early MRCPĎs between MRCP 1 and MRCP 2. In addition MRCPís during Termination could be characterized by right posterior parietal localization. In order to avoid terminological confusion we reserve the term "Initiation" for the "Initiation of Execution" whereas the "initiation of Termination" will be subsumed unter the term "Termination". Third "execution" and "termination" do involve different movements (lifting and lowering) which is reflected in distinct movement potentials in MRCP 1 and MRCP 2. Fourth the "Termination" of movements seems to be particularly related to right parietal cortical function and gaba-ergic neurotransmission since otherwise there would have been no differences between MRCP 1 and MRCP 2 in parietal cortical dipole source location and reaction to lorazepam.
In summary "Termination" of movements may be characterized
by two distinct aspects, initiation and execution, which can be charaterized
by involvement of right parietal cortical function and gaba-ergic neurotransmission.
Furthermore on-line monitoring of the spatial position of the ongoing movement,
as related to right parietal cortical function, may be considered as essential
for "Termination" distinguishing it from "Plan"/"Strategy", "Initiation",
and "Execution".
3.6.3. Termination in catatonia
Kinematic measurements during "Initiation" and "Termination" of finger tapping showed that catatonic patients needed significantly longer for "Termination" than psychiatric and healthy controls whereas in "Initiation" no significant differences between groups were found (Northoff et al. 2000, Pfennig et al. 2000). These results contrasts with those in patients with Parkinson's disease who needed significantly longer time duration for "Initiation" but not for "Termination".
Catatonic patients showed no abnormalities in MRCP's of "Initiation" i.e. lifting (MRCP 1) whereas they showed significantly delayed onsets in early MRCPĎs in central and parietal electrodes during "Termination" i.e. lowering (MRCP 2) compared to psychiatric and healthy controls (Northoff et al. 2000, Pfennig et al. 2000). The fact that the early onset was altered only in MRCP 2 but not in MRCP 1 indicates a delay specifically in "initiation of termination" while "Initiation" itself seems to remain intact. In addition catatonic motor and behavioral symptoms correlated significantly with delayed early onset in MRCP 2 in parietal electrodes. This is further supported by results from dipole source analysis showing alterations in dipole source localization in right posterior parietal cortex in catatonic patients compared to psychiatric and healthy controls.
In summary posturing in catatonia can be characterized
by a specific deficit in "Termination" of movements while "Plan"/"Strategy",
"Initiation", and "Execution" remain basically intact. Such a deficit in
"Termination" of movements is underlined by kinematic and electrophysiological
measurements demonstrating alterations in temporal duration, onset of early
MRCPís, right parietal cortical localization, and gaba-ergic reactivity
in MRCP's specifically related to "Termination" of movements. Delayed onsets
in late MRCPís during Initiation and delayed onsets in early parietal MRCPís
during Termination may reflect a delay in right parietal cortical on-line
monitoring of the spatial position of movements.
3.7. Neurochemical findings
3.7.1. GABA
Recent interest on neurochemical alterations in catatonia has focused on GABA-A receptors because the GABA-A receptor potentiator lorazepam is efficacious in 60-80% of all acute catatonic patients (Rosebush et al. 1990, Bush et al. 1996, Northoff et al. 1995). One study investigated Iomazenil-binding, reflecting number and function of GABA-A receptors, in 10 catatonic patients in Single Photon Emission Computerized Tomography (SPECT) and compared them with 10 non-catatonic psychiatric controls and 20 healthy controls (Northoff et al. 1999). Catatonic patients showed significantly lower GABA-A receptor binding and altered right-left relations in left sensorimotor cortex compared with psychiatric and healthy controls. In addition, catatonic patients could be characterized by significantly lower GABA-A binding in right lateral orbitofrontal and right posterior parietal cortex correlating significantly with motor and affective (but not with behavioral) catatonic symptoms.
Furthermore emotional-motor stimulation in FMRI/MEG (see above) was performed after neurochemical stimulation with lorazepam (see Northoff et al. 2000, Richter et al. 2000). After lorazepam healthy subjects showed a shift of activation from orbitofrontal cortex to medial prefrontal cortex resembling the pattern of activity from catatonic patients before lorazepam (see above). Catatonic patients in contrast showed a reversal in activation/deactivation pattern after lorazepam: Activation in medial prefrontal cortex was replaced by deactivation and deactivation in lateral prefrontal cortex was replaced by activation. It was concluded that prefrontal cortical activation/deactivation pattern during negative emotional processing may be modulated by GABA-A receptors.
In addition to FMRI and MEG kinematic measurements and movement-related cortical potentials were investigated in catatonic patients before and after lorazepam showing abnormal and inverse electrophysiological reactivity (Northoff et al. 2000). After injection of the GABA-A potentiator lorazepam time duration for "Termination" reversed between groups and was now significantly shorter in catatonic patients than in psychiatric and healthy controls. In contrast no influence of lorazepam was observed on temporal duration of "Initiation" in either group.
After lorazepam the early onset in parietal electrodes in MRCP 2 was reversed between groups and was significantly earlier in catatonics than in psychiatric and healthy controls. Lorazepam thus ānormalizedĎ i.e. shortened delayed onset in early MRCPís during Termination in catatonia whereas it delayed early onsets in both psychiatric and healthy controls. Hence the GABA-A potentiator lorazepam lead to reversal in temporal duration of "initiation of termination" in catatonia compared to both control groups. In contrast to MRCP 2 lorazepam had no abnormal influence on MRCP 1 in catatonic patients (Northoff et al. 2000, Pfennig et al. 2000). It should be noted that during neurochemical stimulation with lorazepam all catatonic patients even in a postacute state showed a paradoxical reaction to lorazepam reacting with agitation rather than with sedation as it was the case in all psychiatric and healthy controls.
In contrast to catatonia gaba-ergic transmission in orbitofrontal and prefrontal cortex does not seem to reveal any abnormalities in Parkinsonís whereas they may be subcortical gaba-ergic alterations in basal ganglia.
In summary catatonia can be characterized by major alterations
and abnormal reactivity of GABA-A receptors in right orbitofrontal, motor
cortex, and right parietal cortex apparently leading to abnormal activation/deactivation
pattern and electrophysiological responses whereas in Parkinsonís no such
gaba-ergic abnormalities can be observed.
3.7.2. Dopamine
In early studies Gjessing (1974) found increased dopaminergic (homovanillic acid and vanillic acid) and adrenergic/noradrenergic (norepinephrine, metanephrine, and epinephrine) metabolites in the urine of acute catatonic patients with periodic catatonia. In addition, he found correlations between vegetative alterations and these metabolites. He suggested a close relationship between catatonia and alterations in posterior hypothalamic nuclei. Recent investigations of the dopamine metabolite, homovanillic acid, in the plasma of 32 acute catatonic patients showed increased levels in the acute catatonic state (Northoff 1996) and particularly in those catatonic patients responding well to lorazepam (Northoff 1995). However, the dopamine agonist, apomorphine, exerted no therapeutic effect at all in acute catatonic patients (Starkstein et al. 1996). However asusmption of hyperactivity of the dopaminergic system contradicts with the observation of induction of catatonia by neuroleptics (i.e. "neuroleptic-induced catatonia") which suppress dopaminergic metabolism and should be therapeutic. Therefore most authors (see Caroll 2000) assume that catatonia can be characterized by striatal hypodopaminergia.
In contrast to catatonia dopamine is the major transmitter affected in Parkinsonís. Several studies showed decreased striatal D2-receptor binding in parkinsonian patients.
In summary there are rather inconsistent results with
regard to dopaminergic involvement in catatonia most authors currently
assuming striatal hypodopaminergia since catatonia can be induced by neuroleptics
i.e. a so-called "neuroleptic-induced catatonia". In contrast Parkinsonís
can be characterized by major alterations in nigrostriatal dopamine as
it is reflected in several reduction of D-2 receptors in striatum.
3.7.3. Glutamat
The glutamatergic system, in particular the NMDA-receptors, may also be involved in catatonia. Some catatonic patients (non-responsive to lorazepam) have been successfully treated with the NMDA-antagonist amantadine. Therapeutic recovery occurred rather gradually and delayed (Northoff et al. 1997). Such gradual and delayed improvement suggests that NMDA-receptors may be involved secondarily in catatonia whereas GABA-A receptors seem to be primarily altered. This assumption is speculative since neither the NMDA-receptors nor their interactions with GABA-A receptors have been investigated in catatonia.
In Parkinsonís a modulation of glutamatergic-mediated cortico-striatal pathway by NMDA-antagonists has been suggested as a model for explanation of therapeutic efficiacy of amantadine/memantine (Merriol et al. 1999). Alternatively modulation of glutamatergic pathway within basal ganglia i.e. between subthalamic nuclei and internal pallidum has been discussed to be the major focus of efficiacy.
In summary catatonia and Parkinsonís may be characterized by glutamatergic abnormalities
especially in NMDA-receptors since amantadine s a NMDA
antagonist is therapeutially effective in both diseases. Amantadine may
modulate glutamatergic-mediated cortical and subcortical connectivity.
3.7.4. Serotonine
The serotoninergic system may play a role in catatonia as well since atypical neuroleptics have been shown to induce catatonic features (Caroll 2000). It has been hypothesized that catatonia may be characterized by a dysequilibrium in the serotonergic system with up-regulated 5-HT1a receptors and down-regulated 5-HT2a receptors (Carroll 2000). However there are no imaging studies with regard to the serotonergic system in catatonia so that this hypothesis remains speculative.
Similar to catatonia the serotoninergic system has been found to be involved in Parkinsonís which may be related with dopaminergic abnormalities.
In summary serotoninergic system seems to be involved
and thus altered in both catatonia and Parkinsonís which may reflect secondarily
modulation by primarily altered transmitter systems i.e. GABA in catatonia
and Dopamine in Parkinsonís.
4. PATHOPHYSIOLOGICAL HYPOTHESIS
4.1. Pathophysiology of motor symptoms
4.1.1. Deficit in "Execution" of movements: Akinesia
Both catatonia and Parkinsonís can be characterized by akinesia which may be related with functional alterations in the so-called "direct" "motor loop" ranging from MC/SMA to putamen, from putamen to internal pallidum, and from there via mediodorsal thalamic nuclei back to MC/SMA (Masterman and Cummings 1997). Decrease in striatal dopamine leads to down-regulation of the "direct" "motor loop" (exclusion of external pallidum) and concomittant "up-regulation" of the "indirect" "motor loop" (inclusion of external pallidum) resulting in a netto-effect of decreased activity in premotor/motor cortex.
In contrast to Parkinsonís disease functional imaging studies during performance of movements yielded no alterations in SMA and MC in catatonia. However effective connectivity ranging from orbitofrontal cortex to premotor/motor cortex was significantly reduced during emotional-motor stimulation in catatonic patients. Premotor/motor cortical function does remain apparentely intact during isolated motor stimulation whereas it seems to become dysregulated during emotional stimulation via cortico-cortical connectivity in orbitofrontal/prefrontal cortex. Consequently the "motor loop" itself seems to remain intact in catatonia whereas it is dysregulated by orbitofrontal and prefrontal cortex via "cortico-cortical i.e. horizontal modulation".
In summary akinesia is closely related to down-regulation
of the "motor loop" either by dopaminergic-mediated subcortical-cortical
"bottom-up modulation", as in Parkinsonís, or by gaba-ergic mediated "cortico-cortical
i.e. horizontal modulation" with consecutive "top-down modulation", as
in catatonia.
4.1.2. Deficits in "Initiation" of movements: Starting problems
Parkinsonian patients could be characterized by deficit in initiation which may be considered as one essential component of the "willed action system".
Movements have to be planned and a strategy has to be made in order to get an idea what kind of movement shall be performed which may be closely related to lateral orbitofrontal function (Deecke 1996). This aspect will be referred to as "Plan/Strategy" of movements in the further course of the manusript. There must be an idea of how to move including a decision to perform a movement which can be initiated either internally (i.e.voluntary) or rather externally (i.e. involuntary). Internally initiated movements can be considered as willed movement/actions which may be subserved by a so-called "willed action system" involving the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate, the anterior supplementary motor area (SMA), and fronto-striatal circuits (Jahanshahi et al. 1995, Jahanshahi and Frith 1998, 494, 517-9; Deecke 1996). This aspect will be referred to as "Initiation" in the further course of the manuscript. Once a movement is initiated it can be executed which probably is closely related to function of posterior SMA and the motor cortex (Deecke 1996, Jahanshahi and Frith 1998) which will be referred to as "Execution" in the further course of the manuscript. The executed movement can be characterized by dynamic and kinematic properties. Dynamic properties refer to force and velocity of the movements which may be encoded primarily in neurons of the motor cortex (Dettmers et al. 1995). Fronto-mesial structures such as the SMA as well as the putamen and the ventrolateral thalamus may be important for coding of temporal properties i.e. the 'timing' of movements (Deecke 1996, Jahanshahi and Frith 1998, 493). Kinematic properties describe spatial characteristics of movements such as angles, etc. which may be encoded by neurons in parietal cortex (area 5, 39, 40) (Kalaska et al. 1996, Jeannerod 1997, 57-8, 72-3). Finally the movement must be terminated which will be referred to as "Termination" implying postural change with on-line monitoring of the spatial position of the movement.
Parkinsonís can be characterized by severe deficits in SMA which, as part of the "willed action system", is closely related to the ability of "Initiation". Parkinsonian patients do show indeed severe deficits internal initiation while they are well able to execute them once they have overcome their initiation problems. Consequently Parkinsonís may be characterized by disturbance in the "willed action system" with problems in the voluntary generation of movements by itself (Jahanshahi and Frith 1998).
In contrast to Parkinsonís catatonia cannot be characterized by primary alterations in the "willed action system" since both "Initiation" and function of SMA seems to remain more or less intact in such patients. Therefore voluntary generation and "initiation" implying that the "willed action system" itself remains basically intact. Instead the "willed action system" becomes dysregulated by cortico-cortical connectivity so that it seems as if there is a deficit in "Initiation" in catatonia.
In summary "initiation" as part of the "willed action
system" is disturbed in Parkinsonís accounting clinically for starting
problems whereas in catatonia the intact functioning "willed action system"
becomes dysregulated by cortico-cortical connectivity resulting in motor
similarity between catatonic and parkinsonic patients.
4.1.3. Deficit in "Termination" of movements: Posturing
In order to terminate a movement on-line of monitoring of the spatial position of the respective movement is necessarily required which, neuropsychologically, may be subserved by visuo-spatial attention as closely related to function of right posterior parietal cortex.
The posterior parietal cortex has been shown to be specifically involved in location and direction of the spatial position of movements and limbs in relation to intrapersonal space of the body (Roland et al. 1980, Colby and Duhamal 1996, Anderson 1999). On the basis of spatial attention with a redirection to extrapersonal or sensory space movements will be selected in orientation on the respective spatial context. Providing the spatial frame of reference, the posterior inferior parietal cortex, as in contrast to the posterior superior parietal cortex, is specifically involved in abstract spatial processing and exploration (Karnath 1999). As such the right posterior inferior parietal cortex may provide the intrapersonal "spatial frame of reference of the body necessary for the conscious organization of movements thus making spatial codes available for prefrontal cortical representation" (Vallar 1999; p.45). In addition to spatial monitoring the posterior inferior parietal cortex seems to be specifically involved in early initiation of movements (Castiello 1999, Desmurget et al. 1999, Mattingley et al. 1998, Snyder et al. 1997, Driver and Mattingley 1998) which, in the present context, may be interpreted as a specific relationship between "initiation of Termination" and posterior inferior parietal cortical function. Consequently posterior inferior parietal cortical function may provide the linkage between spatial registration as "internal spatial monitoring" and "initiation of Termination" as necessarily required for postural change and consecutive "execution of Termination".
In catatonia alterations in right parietal cortical functions in catatonia were found in neuropsychology, showing deficits in visuo-spatial abilities correlating with attentional function, and SPECT, showing decreased r-CBF in right parietal cortex and abnormal correlations with visuo-spatial abilities. Involvement of right posterior parietal cortex in pathophysiology of catatonia is further supported by consideration of anatomo-functional parcellation in this region. Distinct areas respresenting eye movements, arm movements, and head movements, may be distinguished within posterior parietal cortex (Colby and Duhamel 1996, Anderson 1999). Such distinct representational areas for eyes, head, and arm coincide with clinical observations that posturing in catatonia can occur in eyes, arms, and/or head. Posturing of eyes may be reflected in staring, posturing of head is reflected in "psychic pillow", and posturing of arm is the classical type of posturing (see above). All three kinds of posturing can occur simultaneously but they may also dissociate from each other so that, for example, patients may show only the "psychic pillow" without staring and posturing of limbs. Such a dissociation between the three kinds of posturing may have its physiological origin in anatomo-functional parcellation in posterior parietal cortex.
Consequently it may be hypothesized that deficit in right parietal visuo-spatial attention in catatonic patients leads to an inability in "initiation of Termination" since spatial position of the ongoing movement can no longer be registrated in an appropriate way. This may result in an inability of "execution of Termination" with a consecutive blockade in postural change which clinically is reflected in posturing. Assumption of relation between posturing as a blockade in postural change and right parietal cortical dysfunction is supported by electrophysiological findings during postural change (Northoff et al. 2000, Pfennig et al. 2000) as well as by observation of posturing in patients with isolated lesions in right parietal cortex (Fukutake et al. 1993, Saver et al. 1993).
Due to additional disturbances in orbitofrontal cortex catatonia has to be distinguished from disorders related with isolated lesions in right parietal cortex as, for example, neglect showing the following differences: (i) patients with neglect do not show posturing; (ii) unlike patients with neglect catatonic patients do neither deny the existence of limbs or parts of their body nor overlook these body parts in relation to the environment so that they do not strike with these body parts against walls, doors, etc.; (iii) patients with neglect do show attentional deficits whereas in catatonic patients no such deficits could be found; (iv) patients with neglect do often show sensory deficits which cannot be observed in catatonia; (v) unlike patients with neglect catatonic patients do not show a right-left pattern with regard to their symptoms i.e. posturing; (vi) unlike patients with neglect catatonic patients do not suffer from alterations in peripersonal and extrapersonal space (as reflected in successfull ball-experiments; Northoff et al. 1995) whereas they may be characterized by alterations in personal space being unable to locate the position of his/her own limbs in relation to the rest of the body. Since personal and peri/extrapersonal space may be subserved by distinct neural networks (Galatti et al. 1999) distinction between both kinds of spaces may not only be phenomenologically relevant but physiologically as well. Hence catatonia cannot be compared with neglect as an attentional disorder so that posturing cannot be accounted for by disturbances in attention which is further supported by neuropsychological findings showing no specific alterations in attentional measures (see above).
Other disorders related with right posterior parietal cortical dysfunction must be distinguished from catatonia as well. Patients with Balint Syndrome show symptoms like an inability to fixate objects and an optic ataxia which both cannot be observed in catatonia. Since Balint Syndrome and especially optic ataxia indicate involvement of right posterior superior parietal cortex differences between catatonia and Balint syndrome do further underline the particular involvement of the right posterior inferior parietal cortex in catatonia.
In contrast to catatonia parkinsonian patients do neither show posturing nor alterations in right parietal cortex.
In summary catatonia can be characterized by specific
deficits in "initiation of termination" while Parkinsonís show rather deficits
in "initiation of execution" implying functional dissociation between both
diseases with regard to initiation of movements. Whereas the deficit in
"initiation of termination" seems to be related with dysfunction in right
posterior parietal cortex lack of "initiation of execution" seems to be
accounted for by functional deficits in SMA.
4.1.4. Alteration in tonus of movements: Cogwheel rigidity and flexibilitas cerea
Parkinsonian patients could be characterized by muscular hypertonus with a so-called "cogwheel rigidity" which may be accounted for by a deficit in striatal D2-receptors and consecutive dyscoordination of activity in internal pallidum.
Catatonic patients do show muscular hypertonus but without "cogwheel rigidity" which is replaced by a smooth kind of rigidity a so-called flexibilitas cerea. Since there is no primary i.e. direct deficit of striatal D-2-receptors in catatonia dyscoordination of the internal pallidum may be not as strong as in Parkinsonís implying that there may be some kind of smooth musuclar hypertonus without cogwheel rigidity. Assumption of discrete down-regulation of striatal D-2 receptors may be supported by symptomatic overlap between catatonia and neuroleptic malignant syndrome, possibility of "neuroleptic-induced catatonia", and central role of striatum in animals models of catatonia (see Caroll 2000).
Origin of down-regulation of striatal D-2 receptors in catatonia remains however unclear. Down-regulation of striatal D2-receptors may be related with cortical alterations: Orbitofrontal cortical alterations may lead to down-regulation of D2-receptors in caudate via "top-down modulation" within the "orbitofrontal cortical loop" (see Figure 4). Or striatal D-2 receptors may be top-down modulated within the "motor loop" which by itself may become dysregulated by cortico-cortical connectivity. However due to lack of specific investigation of basal ganglia in catatonia both assumption remain speculative.
In summary rigidity may be related to alterations in internal
pallidum as induced by down-regulation of striatal D-2 receptors which
may be caused either by subcortical-subcortical connectivity, as in Parkinsonís,
or by abnormal cortico-cortical connectivity with "horizontal modulation"
and concomittant cortico-subcortical "top-down modulation", as it may be
the case in catatonia.
4.2. Pathophysiology of behavioral symptoms
4.2.1. Deficit in on-line monitoring: Motor anosognosia
Subjective experience in catatonic patients could be characterized by unawareness of posturing and of movement disturbances in general whereas parkinsonian patients were well aware of their motor deficits. Consequently question for difference between catatonic and parkinsonian patients with regard to "internal monitoring" of movements arises. It should be noted that catatonic patients showed an unawareness only with regard to their motor disturbances since they well aware or even hyperaware of emotional alterations.
Awareness of movements is closely related to the ability of on-line monitoring as an "internal monitoring" which by itself does necessarily requires generation of an "internal model" of the respective movement. According to Miall and Wolpert (1996), distinct kinds of models can be distinguished (see Figure 2). There is a causal representation of the motor apparatus which can be described as a "Forward dynamic model", there is a model of the behavior and the environment which can be called a "Forward output model", and there is an "Inverse model" inverting the causal flow of the motor system by representing the causal events that produced the respective motor state (for more detailed discussion see Miall and Wolpert 1996).
"Internal monitoring" of movements could itself be either "implicit" or "explicit". Following Jeannerod (1997) only certain aspects of movements are internally monitored in an "explicit" mode of processing. "Plan/Strategy" and to some extent "Initiation" are accessible to consciousness and can thus be characterized by "explicit internal monitoring". In contrast "Execution" itself is not accessible to consciousness and can thus be characterized by "implicit internal monitoring" (Jeannerod 1997). Accordingly Jeannerod distinguishes between an "implicit" "How system" and an "explicit" "Who system" of movements/action the former being responsible for "Execution" whereas the latter includes "Plan/Strategy" and "Initiation".
Empirically such an assumption is further supported by a study from Grafton et al. (1995) investigating whether persons were conscious or non-conscious of a particular order of sequences of movements they performed - consciousness of the order of sequence necessarily presupposing an "explicit internal monitoring" of "Plan/Strategy". Subjects showing consciousness of the order of sequence could be characterized by activation in right dorsolateral prefrontal cortex (Area 9), right posterior parietal cortex (Area 40), and right premotor cortex (Area 6) compared to those subjects who were unconscious. Increasing demand of "explicit internal monitoring", as induced by mirror experiments, lead to activation in right lateral dorsolateral prefrontal cortex (Area 9 and 46) and right posterior parietal cortex (Area 40) (Fink et al. 1999).
Following distinction between "implicit" and "explicit" internal monitoring I want to propose a similar hypothesis for "Termination". It could be distinguished between "initiation of termination" and "execution of termination" emphasizing the particular importance of internal spatial monitoring for "initiation of termination". Following phenomenological accounts of movements one may well be conscious about the spatial position from which one "initiates" the "terminating movement" so that "initiation of termination" may be characterized by "explicit internal monitoring". In contrast "execution of termination" may rather be accompanied by "implicit internal monitoring". Hence spatial position from which the "Termination" is initiated may be accessible to consciousness i.e. "explicit internal monitoring" whereas execution of the terminating movement itself may rather be unconscious and therefore be characterized by "implicit internal monitoring".
"Internal monitoring" of the spatial position of movements may be considered as a subset of on-line monitoring in general which can be considered as a basic and essential component of working memory. On-line monitoring in general is closely related to functional activity in ventrolateral and dorsolateral prefrontal cortex (i.e. VLPFC and DLPFC) (see Petrides 1995, Leary et al. 1999). Therefore it may be hypothesized that on-line monitoring of the spatial position of own movements may be subserved by right-hemispheric network between VLPFC, DLPFC, and posterior parietal cortex (i.e. PPC). Consequently functional connections between right posterior parietal, right dorsolateral prefrontal, and right lateral orbitofrontal/ventrolateral prefronal cortex may be of crucial importance for "implicit" and "explicit internal monitoring" of the spatial position of movements. Thereby, as based on above mentioned studies of motor awareness, the VLPFC seems to be rather related to "implicit internal monitoring" whereas the DLPFC may rather be related to "explicit internal monitoring".
The lateral orbitofrontal/ventrolateral prefrontal cortex shows similar cytoarchitectonic subdivisions as the posterior parietal cortex (Carmichael and Price 1994) and receives reciprocal connections from both posterior parietal and dorsolateral prefrontal cortex which project to similar areas (Selemon and Goldman-Rakic 1988, Cavada and Goldman-Rakic 1989, Morecraft and al. 1992, 1998). In accordance with such reciprocal connectivity co-activation of these three regions has been demonstrated in tasks requiring behavioral flexibility and thus "implicit and explicit spatial monitoring" (Nobre et al. 1999, Quintana and Fuster 1999, Stephan et al. 1999, Athwal et al. 1999, Meyer-Lindenberg et al. 1999). The orbitofrontal cortex may thus modulate activity in dorsolateral and posterior parietal (and other association) cortex which has already been demonstrated in both animals (Quintana et al. 1989) and humans (Drevets and Raichle 1998, Mayberg et al. 1999, Büchel et al. 1997). Furthermore the right orbitofrontal cortex shows a higher density of neurons and neuronal connections which may account for predominance of right hemispheric activation (see below). Consequently the right hemispheric neural network between posterior parietal, dorsolateral prefrontal, and lateral orbitofrontal/ventrolateral prefrontal cortex may be crucially involved in "implicit" and "explicit internal monitoring" of the spatial position of movements and thus in updating of spatial location and representation of movements (Colby 1999).
Catatonia can be characterized by major deficits in on-line monitoring and alterations in right ventro/dorsolateral prefrontal cortex (i.e. VLPFC, DLPFC) and right posterior parietal cortex (i.e. PPC) as has been demonstrated in SPECT and fMRI (see above). Consequently right hemispheric network between VLPFC, DLPFC, and PPC may be altered in catatonia which may account for deficit in on-line monitoring of the spatial position of movements consecutively leading to posturing. One may assume that both kinds of on-line monitoring "implicit" and "explicit internal monitoring" may be deficient in catatonia: Catatonic patients are neither able to terminate their movements, requiring "implicit internal monitoring", nor are they aware of their motor disturbances, requiring "explicit internal monitoring".
Since connection between right VLPFC and right posterior parietal cortex seems to be altered in catatonia on-line monitoring in general becomes deficient. Such disturbance in on-line monitoring in general does not only lead to alteration in "implicit internal monitoring" but in "explicit internal monitoring" since either kind of "internal monitoring" is affected. Consequently deficit in on-line monitoring in general, as related to dysfunction in VLPFC, may account for deficits in "implicit and explicit internal monitoring" of movements and thus for concomittant posturing and motor anosognosia in catatonic patients.
Furthermore one may hypothesize that primary involvement of gaba-ergic transmission may be somehow related to motor anosognosia. Similar to catatonia patients with movement disturbances with primary alteration in GABA, such as Huntington chorea and parkinsonian dyskinesia, do show unawareness of their motor anomalies so that they can be characterized by motor anosognosia as well (Snowdon et al. 1998). However exact relationship between gaba-ergic transmission and motor anosognosia remains unclear.
In contrast to catatonia parkinsonian patients do neither show deficits in on-line monitoring in general nor in "implicit and explicit internal monitoring" of movements in particular. Physiologically this may be reflected in the absence of major deficits of function in VLPFC and gaba-ergic transmission implying that these patients are fully aware of their motor disturbances.
In summary catatonia can be characterized by a ventrolateral
prefrontal cortical dysfunction with a consecutive deficit in on-line monitoing
in general. This deficit may lead to dysregulation of the right-hemispheric
network between VLPFC, DLPFC, and PPC resulting in a deficit in "implicit
and explicit internal monitoring" of the spatial position of movements
which may account for both posturing and motor anosognosia in catatonic
patients.
4.2.2. Deficit in verbal and non-verbal contact: Mutism and Stupor
One of the most impressive clinical features in catatonic patients is mutism or even stupor implying that there is no longer any kind of verbal (mutism) and/or non-verbal (stupor) contact to other persons - neither mutism nor stupor do occurr as such in Parkinsonís.
We showed that alterations in medial and lateral orbitofrontal cortex in catatonia lead to shift of patterns of activity towards anterior cingulate/medial prefrontal cortex and lateral prefrontal cortex during negative emotional processing resulting in functional dysbalance between medial and lateral pathway in prefrontal cortex.
The anterior cingulate (area 24 and 32 according to Brodman) shows anatomical, cytoarchitectonic, connectional, and functional subdivision into an affective (24a), cognitive (24b), and motor (24c) part. Relation between these three subdivision may be characterized by reciprocal suppression (Devinsky 1997): For example strong emotional processing leads to activation of the affective part and concomittant suppression of the cognitive part and vice versa.
Due to shift of patterns of activity from orbitofrontal cortex to anterior cingulate/medial prefrontal cortex during negative emotional processing there may be extremely strong and high activity in the affective part (i.e. 24c) of the anterior cingulate which, via reciprocal suppression, may lead to almost complete down-regulation of functional activity within the motor part of the anterior cingulate. Down-regulation of the motor part in anterior cingulate may account for mutism as an inability to speak and thus to make verbal contact with other persons. Such an assumption would be supported by observation of mutism in patients with isolated lesions in anterior cingulate; in addition such patients can be characterized by combination of akinesia and mutism i.e. akinetic mutism which, off course, is be in accordance with catatonia. However comparison between catatonia and akinetic mutism should be restricted to concomittant occurrence of akinesia and mutism since, unlike in catatonia, patients with akinetic mutism do neither show hyperkinesias nor other behavioral anomalies (like negativism, perseverative and compulsive behavior, etc.).
In addition to anterior cingulate catatonic patients showed functional alterations in medial prefrontal cortex during negative emotional processing as well. The medial prefrontal cortex is involved in social cognition and thus in perception of movements and mental states of other persons (see Castelli et al. 2000). Shift of patterns of activity from orbitofrontal to medial prefrontal cortex may lead to dysfunction of the latter so that the ability to perceive movements and mental states from other persons may be altered as well which, clinically, may account for stupor as an inability to make verbal and non-verbal contact with other persons.
In summary deficit in orbitofrontal cortical activation
during negative emotional processing in catatonia leads to shift of patterns
of activity towards anterior cingulate and medial prefrontal cortex which
may become dysfunctional thereby accounting for mutism and stupor.
4.2.3. Deficit in inhibitory control and planing of behavior: Perseverative-compulsive behavior
In contrast to Parkinsonís catatonia can be characterized by bizarre behavioral anomalies including negativism, stereotypies, perseverations, echolalia/praxia etc. (see above) which may be classified as perseverative and compulsive behavior. These bizarre perseverative and compulsive behavioral anomalies may be closely related to dysfunction in orbitofrontal cortex.
The orbitofrontal cortex and especially the lateral part including the ventrolateral prefrontal cortex (i.e. VLPFC) may be closely related to control and monitoring of complex behavior (Deecke 1996) whereas planing of its details seems to be rather subserved by dorsolateral prefrontal cortical function (i.e. DLPFC) (Jahanshahi and Frith 1998). Thereby control and monitoring of complex behavior may be exerted by inhibition (Dias et al. 1996, 1997) and thus by suppression as an inhibitory control. Similar to VLPFC the DLPFC shows reciprocal connections with posterior parietal cortex (i.e. PPC) (Selemon and Goldman-Rakic 1988, Cavada and Goldman-Rakic 1989) implying that control and monitoring of behavior may be closely associated with registration of the spatial position of the respective movement. Consequently it is the neural network between VLPFC, DLPFC, and PPC which may be closely related to control and monitoring of complex behavior.
Due to deficits in medial and lateral orbitofrontal cortical activation in catatonia the VLPFC may be unable to exert inhibitory control and monitoring of complex behavior. Behavior can thus no longer be controlled by inhibition resulting in lack of suppression of once started behavior with consecutive perseverations. It is such an inability to supress once started behavior which may be reflected in perseverative symptoms like stereotypies, echolalia/praxia, perseverations, etc. Furthermore alterations in lateral orbitofrontal cortex have been shown to be closely related to compulsive behavior, as for example in obsessive-compulsive disorder, which may support our assumption of relation between perseverative-compulsive behavioral anomalies and dysfunction in VLPFC in catatonia.
Dysfunction in VLPFC may lead to functional alteration in DLPFC as well since both are reciprocally connected. In addition to the inability to supress once started behavioral patterns, as related to dysfunction in VLPFC, functional alterations in DLPFC may lead to a deficit in planing the details of new behavior: If one is unable to plan her/his own behavior one has to overtake behavior from other persons by either imitating or negating them as it is reflected in bizarre symptoms like automatic obedience, negativism, echolalia/praxia, mitgehen/machen, etc.. Though assumption of dysfunctional cortico-cortical relation between VLPFC and DLPFC remains speculatively it is supported by findings of significant correlations between behavioral symptoms and lateral orbitofrontal/prefrontal activity during on-line monitoring (see Leschinger et al. 2000).
In summary deficit in orbitofrontal cortex may lead to
concomittant dysfunction in inhibitory control of behavior, as related
with VLPFC, and deficit in planing of new behavior, as related with DLPFC.
Dysfunction in cortico-cortical relation between VLPFC and DLPFC may then
account for perserverative-compulsive behavioral anomalies observed in
catatonia.
4.3. AFFECTIVE SYMPTOMS
4.3.1. Alteration in negative emotional processing: Anxiety
In contrast to Parkinsonís catatonia can be characterized by strong and intense anxieties so that catatonic patients are "paralyzed by fear" or "immobilized by anxieties" (Rosebush et al. 1990, Northoff et al. 1998). Based on such phenomenology a paradigm for emotional-motor stimulation was developped showing a major deficit of activation in medial orbitofrontal cortex during negative emotional processing in catatonia.
The medial orbitofrontal cortex is reciprocally connected with the amygdala i.e. the basal nucleus which is closely related with processing of negative emotions (see Drevets and Raichle 1998, Northoff et al. 2000). Amygdala and medial orbitofrontal cortex have been shown to be activated particuarly during negative emotions whereas both are either less or not activated during positive emotional processing (see Northoff et al. 2000 for an overview).
Processing of negative emotions in medial orbitofrontal cortex seems to be altered in catatonia as characterized by a shift of activation from medial orbitofrontal cortex to anterior cingulate/medial prefrontal cortex (see above). Unfortunately there are no data available yet concerning function of amygdala in catatonia which could potentially further reveal origin of functional deficit in medial orbitofrontal cortex in catatonia.
Occurrence of catatonic syndrome in patients with major depression may give some hints in this regard. Major depression can be characterized by alterations in subgenual area (see Drevets and Raichle 1998, Mayberg et al. 1999) which, via asymmetric amygdalo-prefrontal cortical connectivity (see LeDoux 1996, 287), is closely connected with both medial orbitofrontal cortex and anterior cingulate/medial prefrontal cortex. If dysfunction in subgenual area surpasses a certain threshold, as it may be clinically reflected in strong depressive symptoms, effective connectivity to medial orbitofrontal cortex may be affected as well leading to alteration in orbitofrontal cortical function, as it may be clinically reflected in gradual development of catatonic syndrome in severely depressive patients (see Starkstein et al. 1996). Furthermore amygdala may be affected as well in depression implying that alteration in amygdala may could lead to dysregulation in medial orbitofrontal cortex via modulation of asymmetric connectivity. Consequently there would be at least two other areas i.e. subgenual area and amygdala which, via modulation of effective connectivity, may lead to dysfunction in medial orbitofrontal cortex potentially accounting for clinical observation of occurrence of catatonic syndrome in severely depressive patients. However such a hypothesis remains speculative since currently there are neither data available of subgenual and amygdala function in catatonia nor about thresholds for modulation of cortico-cortical connectivity.
Deficit in medial orbitofrontal cortical activation during negative emotional procesing lead to alteration in balance between medial and lateral pathways in prefrontal cortex. Analysis of structural, functional, and effective connectivity demonstrated that prefrontal cortex may be divided into a medial and lateral pathway (Northoff et al. 2000, Kötter and Northoff 2000): The medial pathway starts from medial orbitofrontal cortex, continues to anterior cingulate and medial prefrontal cortex, and runs into medial premotor cortex (SMA). The lateral pathway starts from lateral inferior prefrontal cortex including lateral orbitofrontal cortex and VLPFC, continues to dorsolateral and upper lateral prefrontal cortex and runs finally into lateral premotor cortex. Both medial and lateral pathway run finally into premotor/motor cortex as a common final functional output station.
Reyling on imaging results and analysis of effective connectivity (Northoff et al. 2000, Kötter and Northoff 2000) negative emotions seem to be predominantly processed in medial pathway in prefrontal cortex whereas positive emotional processing seems to be rather subserved by lateral pathway.
It is the balance between medial and lateral pathway in prefrontal cortex which seems to be altered in catatonia. Catatonia can be characterized by down-regulation of effective connectivity in medial pathway with concomittant upregulation of lateral pathway. Dysfunction in medial prefrontal pathway includes alteration in effective connectivity between medial orbitofrontal cortex and premotor cortex which may account for concomittant occurrence of emotional and motor disturbances in catatonic patients who feel "paralyzed by fear" or "immobilized by anxiety". Due to dysbalance between medial and lateral apthway in prefrontal cortex in catatonia negative emotions may no be processed in an appropriate way resulting, clinically, in overflow of anxiety. Though such an assumption remains rather speculative it is nevertheless supported by findings of highly significant correlations between affective and motor disturbances on the one hand and orbitofrontal and premotor cortical activity on the other (Northoff et al. 2000).
In summary anxiety in catatonia may be related to a deficit
in negative emotional processing in medial orbitofrontal cortex and medial
pathway in prefrontal cortex which may account for concomittant occurrence
of emotional and motor disturbances in such patients.
4.3.2. Deficit in emotional control: Uncontrollability of anxieties
In addition to deficit in medial orbitofrontal cortex during negative emotional processing catatonia can be characterized by functional alterations in lateral orbitofrontal cortex during both negative emotional processing and on-line monitoring (as part of working memory).
Whereas the medial orbitofrontal cortex seems to be closely related to emotional processing the lateral orbitofrontal cortex has been attributed the function of emotional control and attribution of emotional contents to cognitions thereby linking emotional functions with behavior (Carmichael and Price 1994, Damasio 1997, Dias et al. 1996, 1997, Drevets and Raichle 1998, Morecraft et al. 1992, 1998, Shore 1996). Interaction between medial and lateral orbitofrontal cortex provides an "internal model of the social and emotional context" (Bechara 1997, Rolls 1998, Shore, 1996). Such a controlling function in emotional-behavioral processing would be in full accordance with involvement of VLPFC in on-line monitoring in general (see above). Consequently negative emotional processing in medial orbitofrontal cortex may be controlled by on-line monitoring in lateral orbitofrontal cortex implying reciprocal dependence between medial and lateral orbitofrontal cortical function. Such reciprocal dependence between medial and lateral orbitofrontal cortical function may be reflected in a pattern of activation and deactivation found in FMRI (see Baker et al. 1997, Drevets and Raichle 1998, Mayberg et al. 1999, Northoff et al. 2000). Activation (i.e. positively activated activity) in medial orbitofrontal cortex was accompanied by deactivation (i.e. negatively correlated activity) in lateral orbitofrontal cortex whereas activation in lateral orbitofrontal cortex was accompanied by deactivation in medial orbitofrontal cortex.
It is this pattern of activation and deactivation in medial and lateral orbitofrontal cortex which was found to be altered in catatonia as has been revealed in FMRI. If reciprocal dependence between medial and lateral orbitofrontal cortex is altered functional balance between negative emotional processing and emotional control may be altered as well as it is clinically reflected in uncontrollability of anxieties. If the ability of on-line monitoring as a kind of cognitive control of negative emotions is disturbed catatonic patients have to rely on other less sophisticated forms of emotional control, for example, involvement of the motor system (see Northoff et al. 2000). Such a shift from cognitive control to motor control of negative emotional processing may be subserved by network between medial orbitofrontal cortex, lateral orbitofrontal cortex, and premotor/motor cortex (see Figure 3), which was found to be altered in catatonia.
The lateral orbitofrontal cortex can be characterized by close and reciprocal connections with medial temporal lobe encomprising ento/perirhinal structures (Morecraft et al. 1992, 1998, Zald et al. 1998) which may account for occurrence of catatonic syndrome in schizophrenia. Schizophrenia can be characterized by alterations in ento/perirhinal structures (Bogerts et al. 1985, Gray 1995) and if they surpass a certain degree of severity i.e. a certain threshold they may lead to alterations in temporal-lateral orbitofrontal connectivity. Modulation of temporal-lateral orbitofrontal connectivity may account for clinical observation of occurrence of catatonic syndrome in severely affected schizophrenic patients (see Northoff 1997, Northoff et al. 1999).
Dysfunction in balance between medial and lateral orbitofrontal cortex may lead to functional alteration in the network between VLPFC, DLPFC, and PPC (see Figure 3) subserving "implicit and explicit monitoring" of movements and inhibitory control of planing of behavior which, clinically, may account for close relationship between affective and behavioral anomalies in catatonia. Furthermore dysfunction in lateral orbitofrontal cortex may lead to alteration in basal ganglia since, as part of the orbitofrontal loop (see Figure 3), lateral orbitofrontal cortex is closely connected with the ventromedial caudate as part of the striatum which then via pallidum and thalamus connects back to lateral orbitofrontal cortex (see Mastermann and Cummings 1997, Mann et al. 2000). Alteration in lateral orbitofrontal cortex leads to top-down modulation of activity in caudate and other basal ganglia. Such a top-down modulation may potentially account for discrete postmortem findings in basal ganglia (see above) and alterations of r-CBF in caudate in single catatonic patients.
Investigations showed that the VLPFC-DLPFC-PPC network in the right hemishere and not the left hemisphere is altered in catatonia. Since this is based on various neuropsychological and neurophysiological findings there seems to be solid evidence for such a right hemispheric preference. Reasons for such a right hemispheric preference remains however unclear. The predominance of alterations in the right hemisphere (Devinsky 1997), as reflected in our findings, may be accounted for by the fact that orbitofrontal connections with prefrontal and parietal cortex as well as with basal ganglia and the limbic system are much stronger and more expanded in the right cortex than in the left one (Shore 1996, 67). Whether this may account for right hemispheric preference must however remain open. The example of catatonia does nevertheless clearly demonstrates that right hemispheric function can't be transferred to or replaced by left hemispheric function. Furthermore right hemispheric predominance seems to account for both sides of the body i.e. the left and right side since, unlike in neglect patients (see above), there is no lateralization of posturing observable in catatonia (Taylor 1990, Northoff 1997). Finally, as has been demonstrated in recent imaging studies, right hemispheric predominance in orbitofrontal and prefronto-parietal cortical function seems to be related to processes of motor attention (Binkowski et al. 1999) and motor inhibition (Strik et al. 1999) which would be in accordance with our findings in catatonia.
In summary deficit in emotional control and thus the inability
to control anxieties in catatonia may be related to dysfunction in reciprocal
dependence between medial and lateral orbitofrontal cortex. In addition
orbitofrontal cortical dysfunction does lead to dysregulation of both VLPFC-DLPFC-PPC
network and orbitofrontal loop thereby accounting for concomittant occurrence
of emotional and behavioral disturbances in such patients.
4.3.3. Dysfunctional regulation of mood: Depression
The mood can be altered in both catatonia and Parkinsonís: Catatonic patients may develop catatonic syndrome on the basis of preexisting depression whereas parkinsonic patients may develop depression either before (especially older patients) or after manifestation of motor symptoms.
Depression as a dysfunctional regulation of mood has been related with alterations in dorsolateral prefrontal cortex and anterior cingulate in Parkinsonís (Mayberg et al. 1999). Both anterior cingulate and DLPFC are involved in the "willed action system" accounting for planing of movements in detail (see above). Down-regulation of anterior cingulate and DLPFC may thus affect regulation of both mood and movements which potentially may account for concomittant occurrence of depresive and motor features in Parkinsonís. Such an assumption is further supported by consideration of medial prefrontal loop and dorsolateral prefrontal loop connecting anterior cingulate and DLPFC with basal ganglia which may be altered in depressive patients with Parkinsonís (Masterman and Cummings 1997). Reduction of striatal dopamine may down-regulate both anterior cingulate and DLPFC via "bottom-up modulation" within medial prefrontal and dorsolateral prefrontal loop which would be supported by dopaminergic dependency of parkinsonian depression.
Characterization of DLPFC by asymmetric connectivity with strong (feedforward) connections towards premotor/motor cortex and rather weak or even absent (feedback) connections towards orbitofrontal cortex (Kötter and Northoff 2000) may account for the absence of emotional and behavioral abnormalities in Parkinsonís as they can be observed in catatonia. Due to asymmetric connectivity the DLPFC can exert predominantly feedforward effects, resulting in modulation of movements, whereas feedback effects with potential modulation of orbitofrontal cortical function and thus of behavior and emotions by altered movements remain rather weak. Note that the same principle i.e. asymmetric connectivity may account for concomittant occurrence of behavioral, emotional, and motor symptoms in catatonia, since, due to strong feedforward connections, premotor/motor cortical function becomes affected by orbitofrontal cortical dysfunction.
In summary depression in Parkinsonís disease may be accounted
for by down-regulation of DLPFC which, due to asymmetric connectivity,
may affect only premotor/motor cortical function whereas orbitofrontal
cortical function remains unaffected potentially accounting for absence
of major emotional-behavioral anomalies in such patients.
4.4. THERAPEUTIC AGENTS
4.4.1. GABA-ergic agents: Lorazepam
Most (60-80%) catatonic patients show an almost dramatic and immediate therapeutic efficiacy of the GABA-A potentiator lorazepam which as a benzodiazepine shows anxiolytic properties. Imaging studies demonstrated an altered modulation of patterns of activation and deactivation in medial and lateral orbitofrontal cortex during negative emotional stimulation after application of lorazepam (see above) whereas in healthy controls lorazepam lead to an activity pattern similar to the one of catatonic patients without lorazepam. Activity in orbitofrontal cortex may thus be modulated by GABA-A receptors which is supported by findings of dense gaba-ergic innervation in orbitofrontal cortical neurons (Davis 1994, Carmichael and Price 1994). Consequently one has to assume (a deficit and consecutive) dysfunction in GABA-A receptors in orbitofrontal cortex leads to abnormal modulation of reciprocal dependence between medial and lateral orbitofrontal cortex at is reflected in altered patterns of activation and deactivation after application of lorazepam (see Northoff et al. 2000).
Relationship between emotional processing, orbitofrontal cortical function, and GABA-A receptors is supported by several investigations. In an animal model Crestani et al. (1999) showed that gaba-ergic substances i.e. benzodiazepines lead to reversal of anxiety-driven behavior and modulation of activity in GABA-A receptors in prefrontal, amygdala, and hippocampal areas. In healthy humans lorazepam lead to alteration in subjective experience and perception of emotions (Ferrara et al. 1999, Garcia et al. 1997). Benzodiazepines show dramatic therapeutic effects in neuropsychiatric diseases charcterized by orbitofrontal cortical dysfunction and strong anxieties such as obsessive-compulsive disorder (Coplan and Lydiard 1998) and panic disorder (Gorman et al. 2000). Physiologically it has been demonstrated that gaba-ergic agents lead to alterations in r-CBF in rats (Forman et al. 1998) and humans (Mathew et al. 1995, Spanaki et al. 1999, Wang et al. 1995) which may influence neuronal activity as well.
Dysfunction in orbitofrontal cortical GABA-A receptors may lead to regulatory and compensatory changes in sensitivity of GABA-A receptors in VLPFC-DLPFC-PPC network and orbitofrontal-premotor/motor connections which has been supported by various empirical findings (see above). Since gaba-ergic deficits in orbitofrontal cortex seem to alter prefronto-parietal corical networks one may characterize orbitofrontal cortical function as a "gating function".
The orbitofrontal cortex seems to work predominantly via neural inhibition (Dias et al. 1996, 1997, Kiefer et al. 1998, Shore 1996, Strik et al. 1999, Zubicaray et al. 1999). Thereby inhibition may be understood in different senses. First inhibition may be understood in a behavioral sense implying, for example, suppression and thus inhibition of once started behavior or emotions. Inhibition in this sense may certainly be deficient in catatonia as it is reflected in perseverative-compulsive behavior and uncontrollable emotions. Second inhibition may be understood in a functional i.e. connectional sense. For example orbitofrontal cortical activity may lead to suppression and thus inhibition of activity in parietal cortex as it is reflected in desinhibition and increased activity of the latter in the case of orbitofrontal cortical lesions (Jahanshahi and Frith 1998). Since we observed alterations in both orbitofrontal and parietal cortex one may assume alterations of inhibition in a functional i.e. connectional sense in catatonia as well. Third inhibition may be understood in a neuronal sense as opposed to excitation. GABA-A receptors are inhibitory leading to hyperpolarization of nerve cells making induction of action potentials and thus neuronal excitation less likely. This sense of inhibition may be altered in catatonia as well since lorazepam is quite effective. It is important to note that only inhibition in a neuronal sense may be directly related to GABA-A receptors whereas both functional and behavioral inhibition may be mediated by glutamatergic or other transmitter systems.
One may assume that local and autoregulatory gaba-ergic mediated neuronal inhibition may be deficient in orbitofrontal cortex in catatonia which in turn may modulate glutamaergic-mediated connections from orbitofrontal cortex to VLPFC, DLPFC, PPC, and premotor/motor cortex. Such an altered functional i.e. connectional inhibition may go along with alteration in behavioral and emotional inhibition accounting for catatonic symptoms. Only deficit in orbitofrontal cortical neuronal inhibition may be directly related with GABA-A receptors whereas alterations in functional and behavioral inhibition may be related with other transmitter systems (see also Caroll 2000). Such an assumptions does however remain speculatively since studies investigating modulation of effective connectivty by different transmitter systems are still lacking.
In summary gaba-ergic potentiation by lorazepam may compensate
for deficit in gaba-ergic mediated orbitofrontal cortical "gating function".
This may lead to consecutive Ďnormalizationí in cortico-cortical connectivity
via "horizontal modulation" and cortico-subcortical connectivity via "top-down
modulation" which, clincially, may account for almost immediate resolution
of emotional, behavioral, and motor symptoms.
4.4.2. Glutamatergic agents: Amantadine
Amantadine is a NMDA-antagonist leading to down-regulation of glutamatergic-mediated excitation which is therapeutically effective in both catatonia and Parkinsonís disease.
Catatonia could be characterized by a deficit in local gaba-ergic mediated inhibition in orbitofrontal cortex which, via "horizontal modulation" and "top-down modulation", seems to lead to alterations in both prefronto-parietal network and premotor/motor-basal ganglia network. Cortico-cortical association fibres can be characterized by excitatory i.e. glutamatergic transmission and are therefore dependent on NMDA-receptors. Due to deficit in neuronal inhibition as reflected in decreased local gaba-ergic mediated inhibition in orbitofrontal cortex it may be speculated that glutamatergic-mediated connections from orbitofrontal cortex to parietal and premotor/motor cortex may be overexcited consecutively leading to dysfunction in both areas. Amantadine as a NMDA-antagonist may indirectly compensate for lack of gaba-ergic mediated neuronal inhibition by blocking glutamatergic overexcitation in orbitofrontal connections. Instead of direct increase in gaba-ergic mediated neuronal inhibition, as it is the case in immediate therapeutic efficiacy of lorazepam, amantadine does rather indirectly increase neuronal inhibition by down-regulation of neuronal excitation which, clinically, may be reflected in delayed therapeutic response.
Thereby the motor cortex seems to be of crucial importance. Spect investigation showed significantly reduced benzodiazepine binding in catatonia (see above) implying deficit in GABA-A receptor functions which may be related with dysregulation of the "motor loop". The motor cortex seems to show a high density of gaba-ergic neurons and thus of GABA-A receptors which is supported by findings of major reduction in amplitude of movement-related magnetic fields in MEG after application of lorazepam in healthy subjects (Northoff et al. 2000). Assumption of gaba-ergic dependency of cortical motor function is supported by findings of modulation of movements by application of gaba-ergic agents into primate motor cortex (Hikosaka 1985, Kubota et al. 1996, Kurata et al. 1994). Furthermore there seems to be strong and direct interference between GABA-A receptors and NMDA receptors in motor cortex since ketamine as a NMDA-antagonist lead to strong and highly significant alterations in benzodiazepine binding in this region in healthy controls (see Northoff et al. 2000). Consequently one may speculate that amantadine as a NMDA-anatogonist may interact with GABA-A receptors in motor cortex leading to down-regulation of glutamatergic-mediated overexcitation which may account for gradual and delayed resolution of catatonic motor symptoms.
Schizophrenia may be characterized by glutamatergic alterations in NMDA-receptors and consecutive overexcitation in prefrontal cortical and medial temporal areas (Abi-Saab et al. 1998, Olney and Farber 1995). If, due to glutamatergic connections from medial temporal areas, activity in lateral orbitofrontal/prefrontal cortex becomes dysregulated in schizophrenia, it may well be imaginable that, surpassing a certain degree of severity of alteration in functional connections, glutamatergic overexcitation may spread to other cortical areas such as, for example, to posterior parietal cortex and premotor/motor cortex. Clinically such a cortico-cortical spread of glutamatergic-mediated overexcitation may be reflected in occurrence of catatonic syndrome in schizophrenia.
In addition to glutamatergic mediated cortico-cortical connections amantadine may modulate cortico-subcortical and subcortico-subcortical connections which are mediated by NMDA-receptors as well. Cortico-subcortical connections from premotor/motor cortex to striatum i.e. putamen and subcortical-subcortical connections from subthalamic nucleus to internal globus pallidus are modulated by glutamate and thus by NMDA-receptors. It is assumed that, in Parkinsonís, either of these connections or both are modulated by the NMDA-antagonist amantadine leading to gradual resolution of parkinsonian symptoms. It should be noted that, in addition to NMDA-antagonism, amantadine has weak dopamine release and agonist properties which could account for therapeutic efficiacy as well (see Caroll 2000).
In summary amantadine may be therapeutically efficiaous
in catatonia by down-regulation of glutamatergic-mediated overexcitation
in both cortico-cortical and cortico-subcortical connectivity. In Parkinsonís
amantadine may lead to top-down modulation of glutamatergic mediated cortico-striatal
connectivity and/or modulation of subcortical connectivity within the basal
ganglia themselves.
4.4.3. Dopaminergic agents: L-Dopa and neuroleptics
Parkinsonís can be characterized predominantly by deficit or/and down-regulation of D-2 receptors in striatum which therapeutically may be compensated for by dopaminergic agents such as L-Dopa. Functional compensation of decreased striatal D-2 receptor function restores functional balance between indirect and direct motor loop (see Mastermann and Cummings 1997) with reinforcement of the latter and concomittant weakening of the former resulting in a netto-effect of increased excitatory activity in premotor and motor cortex via "bottom-up modulation". In addition to the "motor loop", which is regulated by nigrostriatal dopaminergic system, medial prefrontal and dorsolateral prefrontal loops, as regulated by mesocortical dopaminergic system, may be restored as well by dopaminergic agonists.
D-2 receptor antagonists as typical neuroleptics such as, for example, haloperidol lead to blockade and thus down-regulation of striatal D-2 receptors which may worsen parkinsonian symptoms so that typical neuroleptics should be avoided in parkinsonian patients.
In addition typical neuroleptics may induce catatonic syndrome so that some authors speak of "neuroleptic-induced catatonia" in depressive or schizophrenic patients (Fricchione et al. 2000). Neuroleptics such as haloperidol down-regulate striatal D-2 receptors which, via bottom-up modulation, may lead to reinforcement of already preexisting cortical alterations in depressive and schizophrenic patients. Combination of deterioration in cortical function and concomittant subcortical D-2 blockade may then induce alterations in cortico-cortical and cortico-cortical connectivity via "horizontal modulation" and "vertical modulation" being more or less similar to catatonia i.e. "neuroleptic-induced catatonia. It should however be noted that, in addition to D-2 receptors other dopaminergic receptors i.e. D1 and D4/5 may be involved in catatonia as well.
In summary dopaminergic agonistic agents such as L-Dopa
compensate for deficiency in striatal D-2 receptors in Parkinsonís leading
to "normalization" of functional activity in premotor/motor cortex via
"bottom-up modulation".
5. CONCLUSION
We compared clinical symptoms, neuropsychology, and pathophysiology
between Parkinsonís and catatonia both revealing similarities and differences
so that Parkinsonís may be characterized as a motor disorder and catatonia
rather as a psychomotor disorder. Comparison revealed nature of cortico-subcortical
relations, reflecting "top-down and bottom-up modulation as forms of "vertical
modulation", and cortico-cortical relations reflecting a form of "horizontal
modulation".
5.1. What is "top-down modulation"?
Within the present framework top-down modulation may be described as a modulation of subcortical structures by cortical areas as it is, for example, reflected in modulalation of caudate and other basal ganglia by lateral orbitofrontal cortex (see Figure 4). Such a top-down modulation has to be distinguished from bottom-up modulation as a modulation of cortical areas by subcortical structures as it is, for example, reflected in modulation of premotor/motor cortical areas by basal ganglia via the "motor loop". Consequently cortico-subcortical relations may be characterized by bidirectionality in modulation as it is reflected in concomittatn presence of both "top-down and bottom-up modulation".
In addition to "top-down and bottom-up modulation" as forms of "vertical modulation" one may describe cortico-cortical and subcortico-subcortical modulation as forms of "horizontal modulation" (see Juarrero 1999, 197-9; Hurely 1998, 421).
It should be noted that cortico-cortical connectivity is sometimes one-way implying absence of reciprocal connectivity between two cortical areas. For example (see Edelman and Tononi 2000, 180) pyramidal neurons in layer V in posterior SMA and motor cortex are directly or indirectly related to motor effectors via long range axons traveling through the spinal cord. These neurons are directly connected with neurons in layer VI in anterior SMA and other prefrontal cortical areas which are predominantly related with the thalamo-cortical loop as the main feedback loop. However interaction between neurons in layer V and VI is one-way - there is interaction from layer VI to layer V but no reciprocal interaction from layer V to layer VI. Consequently the thalamo-cortical loop as the main feedback loop may modulate cortical activity in SMA/MC via connections from layer VI to layer V whereas an inverse modulation from layer V to layer VI remains impossible. Therefore cortico-cortical relations as a form of "horizontal modulation" remain unidirectional implying that prefrontal cortical activity may modulate activity in SMA/MC but not vice versa. This is also reflected in absence of direct connections from premotor/motor cortex to dorsolateral and orbitofrontal cortex whereas both orbitofrontal and dorsolateral cortical areas are directly connected with SMA/MC (see Northoff et al. 2000, Kötter and Northoff 2000). Another example of such connectional asymmetry would be connectivity between amygdala and prefrontal cortex which is much stronger in direction from amygdala to prefrontal cortex than from prefrontal cortex to amygdala (LeDoux 1996, 287). Such connectional asymmetry may prevent short-circuiting thereby providing the anatomo-connectional substrate for output orientation which, in the present context, is reflected in premotor/motor cortex as the common final output area for both medial and lateral pathway in prefrontal cortex (see Kötter and Northoff 2000).
In contrast to bidirectionality in "vertical modulation" "horizontal modulation" can, at least in some cases, be characterized by unidirectionality which may be reflected in difference between Parkinsonís and catatonia. Parkinsonís can be characterized by alterations in striatal dopamine which, via "bottom-up modulation" within the "motor loop", leads to down-regulation of activity in SMA/MC accounting for akinesia as a motor symptom. However, probably due to connectional asymmetry in cortico-cortical relations implying unidirectionality in modulation down-regulation of activity in SMA/MC does not necessarily lead to alteration in prefrontal cortical activity as it is reflected in absence of "horizontal modulation" and thus of behavioral and affective symptoms in such patients. Catatonia, in contrast, can be characterized by concomittant motor and affective-behavioral symptoms which may be related to alterations in both "top-down modulation", potentially accounting for motor symptoms, and "horizontal modulation", potentially accounting for affective-behavioral symptoms. Consequently difference in clinical symptoms between Parkinsonís as a motor disorder and catatonia as a psychomotor disorder may be related with connectional asymmetry in cortico-cortical relations implying unidirectionality in "horizontal modulation".
In summary difference between Parkinsonís and catatonia
with regard to involvement of affective and behavioral disturbances may
be related with difference in directionality between "vertical modulation"
and "horizontal modulation" the former showing bidirectionality whereas
the latter may rather be characterized by unidirectionality.
5.2. Where can "top-down modulation" be located?
Both "top-down and bottom-up modulation" take place within functional systems which can be described in the following way: "According to this view a function is, in fact, a functional system (....) directed towards the performance of a particular biological task and consisting of a group of interconnected acts that produce the corresponding biological effect. The most significant feature of a functional system is that, as a rule, it is based on a complex dynamic Ďconstellationí of connections, siuated at different levels of the nervous system, that, in the performance of the adpative task, may be changed with the task itself remaining unchanged" (Luria 1966, preface).
For example the "motor loop" allowing for both "top-down and bottom-up modulation" can be regarded as an essential part of the "willed action system" (see Jahanshahi and Frith 1998) which may be considered as a functional system in the sense of Luria. The "willed action system" can be characterized by "functional circuits", allowing for "top-down and bottom-modulation", and "functional configurations", as reflected in certain patterns of activity across the various regions involved in the respective "functional system". Parkinsonís can be characterized by a disturbance in the "willed action system", as it is reflected in alteration of "vertical modulation" i.e. bottom-up modulation accounting for the deficit in initiation of movements. In contrast to Parkinsonís catatonia can not be characterized by disturbance of the "willed action system" - instead the "willed action system" itself remains intact but becomes dysregulated by cortico-cortical connectivity and thus by "horizontal modulation". Gaba-ergic disturbance in orbitofrontal cortex seems to lead to dysregulation in SMA/MC activity via "horizontal i.e. cortico-cortical modulation" with consecutive dysregulation of the "willed action system" accounting for concomittant motor and affective-behavioral symptoms in such patients.
In contrast to "vertical modulation", which takes place within a particular functional system, "horizontal modulation" can rather be located between different functional systems. Relation between emotions, as subserved by medial pathway in prefrontal cortex, behavior, as subserved by VLPFC-DLPFC-PPC network, and movements, as subserved by network between premotor/motor cortex and basal ganglia, seems to be altered in catatonia which may be traced back to disturbances in cortico-cortical connectivity reflecting alteration in "horizontal modulation".
In summary difference between Parkinsonís as a motor disorder
and catatonia as a psychomotor disorder may be related to difference between
"vertical modulation" and "horizontal modulation" with regard to location.
"Vertical modulation" can be located within specific functional systems
whereas "horizontal modulation" intermediates between different functional
systems.
5.3. When does "top-down modulation" becomes visible?
Both "top-down and bottom-up modulation" become visible only in case of alterations of the respective functional system. In the case of Parkinsonís dopaminergic deficit in striatum makes adjustment in activation of SMA/MC via "bottom-up modulation" within the "motor loop" necessary which can compensate for up to 80% reduction of striatal dopamine. As long as it can fully compensate for striatal deficits alteration in "bottom-up modulation" does not become visible which however changes with the appearance of the first clinical symptoms. In the case of catatonia alteration in "top-down modulation" becomes visible only if catatonic patients show motor symptoms more or less similar to the ones in Parkinsonís as it is clinically reflected in manifestation of akinesia and rigidity. Functionally such motor similarity may be reflected in alteration of the same "functional circuit" i.e. "motor loop" in both diseases: The "motor loop" may either be "top-down modulated" by cortical alterations, as it is the case in catatonia, or "bottom-up modulated" by subcortical alterations, as it is the case in Parkinsonís. Consequently the "motor loop" can be altered via "top-down and bottom-up modulation" by both alterations in cortical areas and subcortical structures which comes close to what P.Schilder called the "principle of double way": "The fact that function of the same anatomical apparatus may be disturbed by both organic lesions and psychological alterations can be described as the Ďprinciple of double wayí" (Schilder 1925, 81; translation by myself). Clinically the "principle of double way" may be reflected in "double-facedness of psychomotor function" ("Doppelgesichtigkeit der Psychomotorik"; Homburger 1932, 261) implying that motor symptoms, as for example akinesia, may either be of neurologic or psychiatric origin.
However, from a functional point of view, "top-down modulation" is not the same as "bottom-up modulation". Functional difference between "top-down and bottom-up modulation" may be reflected in slight differences in motor symptoms: Akinesia in Parkinsonís does go along with deficits in initiation whereas akinesia in catatonia is closely related to a deficit in termination so that the same sign i.e. akinesia may be accompanied by different symptoms in both diseases respectively i.e. starting problems and posturing. Another example would be rigidity describing muscular hypertonus: Parkinsonian patients typically show cogwheel rigidity whereas catatonic patients can rather be characterized by a smooth kind of rigidity i.e. flexibilitas cerea. Though exact pathophysiological mechanisms underlying both kinds of rigidity remain unclear it may nevertheless be speculated that symptomatic differences may be related to functional difference between "top-down modulation" and "bottom-up modulation".
In summary "top-down and bottom-up modulation" within
the same functional system may account for apparent similarities between
Parkinsonís and catatonia with regard to motor symptoms whereas functional
difference between "top-down modulation" and "bottom-up modulation" may
be reflected in slight differences in presentation of motor symptoms.
5.4. How is "top-down modulation" implemented?
Both "top-down and bottom-up modulation" as well as "horizontal i.e. cortico-cortical modulation" are implemented by means of functional i.e. effective connectivity reflecting both feedforward and feedback connections which may regulate and modulate functional relation between cortical areas and cortical areas/subcortical structures. Relying on particular "thresholds" "functional clusters" (see Edelman and Tononi 2000, 146 for the use of the terms "thresholds" and "functional clusters" in the present sense) reflecting certains patterns of activity across different cortical/subcortical regions may be generated via "vertical modulation" which then may account for the respective functional systems in the above mentioned sense. These "thresholds" may be modulated by different transmitter systems. For example, dopamine especially D-2 receptors seem to be essential for modulation of the "willed action system" since, as in Parkinsonís, deficits in striatal dopamine lead to disturbance in functional balance between basal ganglia and premotor/motor cortex. Application of dopaminergic substances does apparently alter the "threshold" for "bottom-up modulation" between basal ganglia and premotor/motor cortex which clinically is reflected in resolution of motor symptoms.
In catatonia gaba-ergic deficits in orbitofrontal cortex seem to modulate the "threshold" for "horizontal modulation" as it may be reflected in alteration of cortico-cortical effective connectivity and thus in different "functional clusters" in prefronto-parietal cortical networks. Application of gaba-ergic substances does apparently restore "horizontal modulation" and thus prefronto-parieto cortical "functional clusters" resulting in consecutive resolution of catatonic symptoms.
In both cases local changes in specific transmitters/receptors do lead to alterations in functional/effective connectivity which may be intermediated by modulation of "thresholds" for "vertical and/or horizontal modulation". Thereby other transmitter/receptors besides dopamine and GABA may be affected as well. Since long fibres connecting different cortical and cortical/subcortical areas are primarily mediated by excitatory transmission i.e. glutamate excitatory transmission may be altered as well in both diseases. This is reflected in therapeutic efficiacy of the NMDA-antagonist amantadine in both catatonia and Parkinsonís. Moreover other transmitter systems such as serotonin may be dysregulated as well since they may be closely connected with gaba-ergic and/or glutamatergic systems.
In summary differences and similarities in therapy between
Parkinsonís and catatonia may be accounted for by alterations in "thresholds"
for different kinds of modulation i.e. "vertical and horizontal modulation"
which are apparently determined by different though overlapping transmitter
systems.
5.5. Why is there "top-down modulation"?
We saw that "vertical modulation" i.e. "top-down and bottom-up modulation" takes place within a particular functional system where apparently it serves for adjustment between distinct functional components within the respective functional system. For example, the "willed action system" can be characterized by distinct components i.e. "Plan/Strategy", "Initiation", "Execution" (and potentially "Termination") from which especially "Initiation" is affected in Parkinsonís. Alteration in "bottom-up modulation" serves for compensation of deficit in "Initiation" which, considering that parkinsonian symptoms do become manifest only after 80% reduction of nigro-striatal dopamine, is functionally quite effective and thus successful.
In contrast to "vertical modulation" being responsible for adjustment of distinct components within one particular functional system "horizontal modulation" may be characterized by adjustment between different functional systems. For example, relation between functional systems subserving behavioral planing/control, negative emotional processing, and generation of movements (i.e. willed action system) seems to be altered in catatonia consecutively leading to symptoms in all three domains.
Adjustment between distinct components within one particular functional system via "vertical modulation" as well as adjustment between different functional systems via "horizontal modulation" may serve ultimately for specific and individual adjustment of the organism to environment. Both "vertical and horizontal modulation" may reinforce particular components within functional systems or certain functional systems while concomittantly and reciprocally suppressing either other components within the same functional systems or other functional systems (Shulman et al. 1997). For example "horizontal modulation" may lead to concomittant activation and deactivation in medial and lateral orbitofrontal cortex implying reciprocal regulation between affect and cognition (see above and Northoff et al. 2000, Drevets and Raichle 1998). Alteration in "horizontal modulation" may disrupt this pattern of concomittant activation and deactivation as it is, for example, the case in catatonia as characterized by abnormal affective-behavioral coupling (see above). "Vertical modulation" may lead to concomittant activation and deactivation in cortical and subcortical areas thereby probably accounting for generation of specific functional systems, as for example for generation and initiation of movements. Dysregulation in "vertical modulation" of this particular functional system does consecutively lead to alteration in initiation of movements as it is reflected in akinesia in Parkinsonís as a disturbance of the "willed action system".
In summary "vertical modulation" serves for adjustment
between distinct components within one particular functional system whereas
"horizontal modulation" seems to account for adjustment between different
functional systems. Ultimately both "vertical and horizontal modulation"
may serve for appropriate i.e. specific and individual adjustment between
organism and environment which may be altered in the case of disturbance
of either kind of modulation as it is reflected in Parkinsonís and catatonia.
FIGURES

--
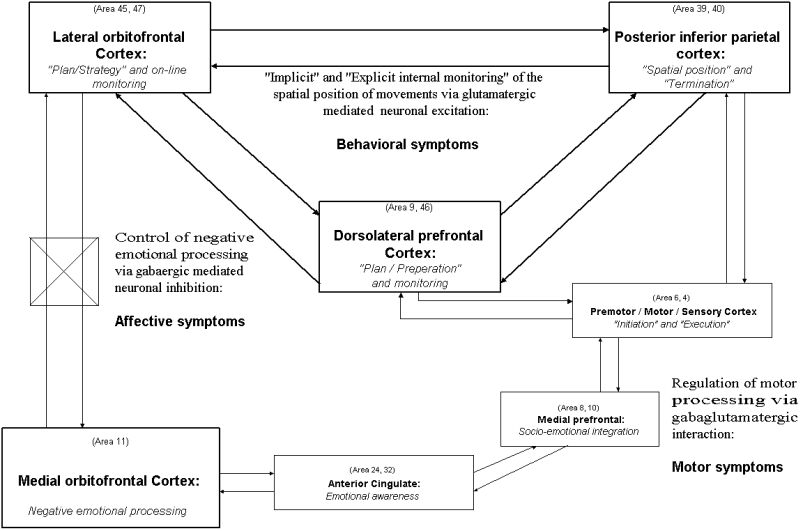
--
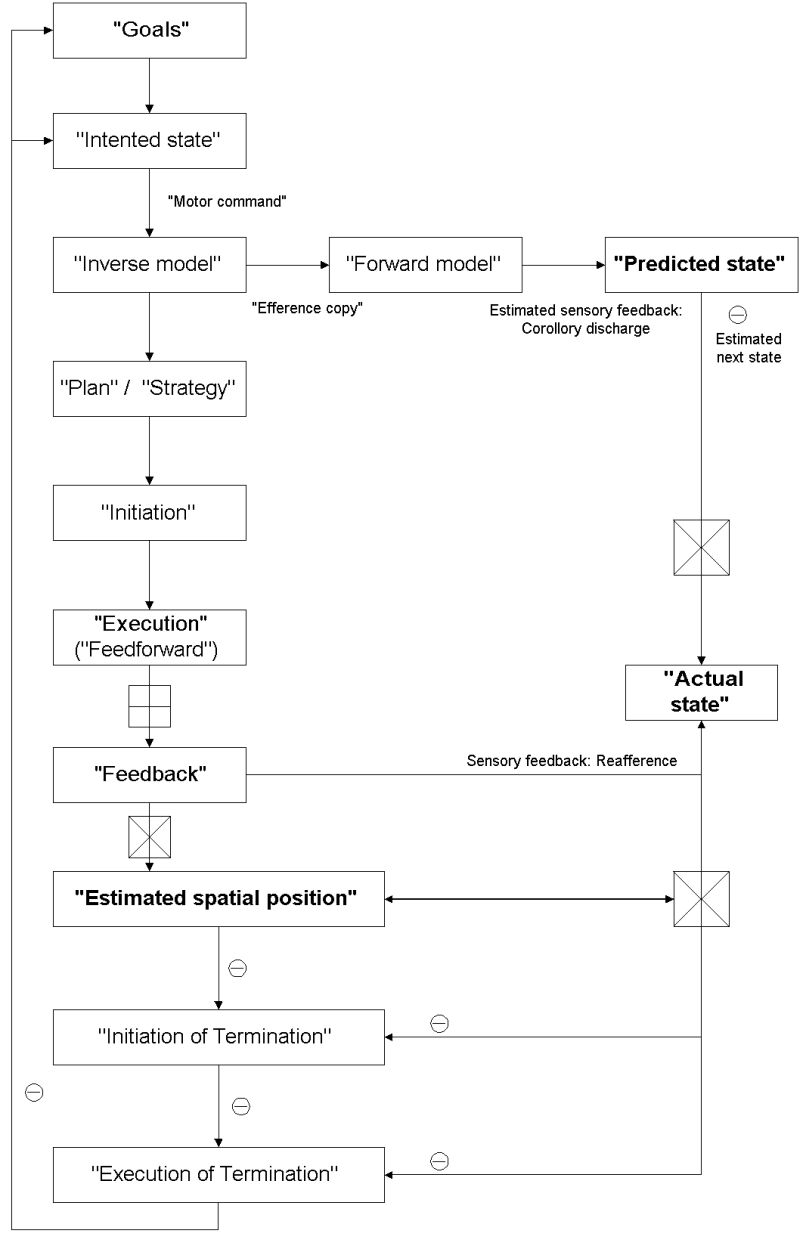
--
Table 1 Neuropsychological and pathophysiological findings
in catatonia and Parkinsonís disease
|
|
|
|
| Neuropsychology | - Visuospatial attention
- On-line monitoring - Emotionally-guided decisions |
- Executive functions |
| Postmortem | - Caudate, N.accumbens, Pallidum, Thalamus | - Substantia nigra, Putamen, Caudate |
| Animal models | - Bulbocapnine, Stress, GABA | - 6-OHDH, MPTP |
| Structural imaging | - Prefrontal and parietal cortex | - Basal ganglia |
| Functional imaging | - Right prefronto-parietal CBF
- Right OFC - Prefrontal connectivity |
- SMA/MC
- Lateral prefrontal cortex - Fronto-striatal connectivity |
| Electrophysiology | - Late and postural RP
- RP modulation by lorazepam |
- Early RP
- RP modulation by dopamine |
| Neurochemistry | - GABA-A receptors
- NMDA receptors - 5 HT1a/2a |
- D-2 receptors in striatum
- NMDA receptors - 5 HT2a |
RP = Readiness Potential
SMA = Supplementary motor area
OFC = Orbitofrontal cortex
MC = Motor Cortex
|
|
|
||
| Motor symptoms | Akinesia | - Cortico-cortical
- Gaba-ergic |
- Subcortico-cortical
- Dopaminergic |
| Starting problems | - Top- down-regulation of SMA/MC | - Deficit in SMA/MC in relation to altered bottom-up modulation | |
| Posturing | - Right orbitofrontal
- Right posterior parietal |
- | |
| Rigidity | - Top-down modulation of striatal D-2 receptors | - Deficit in striatal D-2 receptors | |
| Behavioral symptoms | Motor anosognosia | - Network between ventrolateral, dorsolateral and parietal cortex | - |
| Mutism and Stupor | - Anterior cingulate and medial prefrontal cortex | - | |
| Preservative-compulsive behavior | - Concomittant dysfunction in dorso- and ventrolateral prefrontal cortex | - | |
| Affective symptoms | Anxieties | - Medial orbitofrontal cortex
- Dysbalance between medial and lateral prefrontal cortical pathway |
- |
| Inability to control anxieties | - Dysfunctional relation between medial and lateral orbitofrontal cortex | - | |
| Depression | - | - Anterior cingulate | |
| Therapeutic agents | GABA
(lorazepam) |
- Gaba-ergic mediated neuronal inhibition in
medial orbitofrontal cortex
- Modulation of functional and behavioral inhibition |
- |
| NMDA
(Amantadine) |
- Down-regulation of glutamatergic-mediated overexcitation in prefrontal and orbitofrontal-parietal pathways | - Down-regulation of glutamatergic-mediated overexcitation in subcortical pathways | |
| Dopamin | - Top-down modulation of striatal D-2 receptors predisposing for neuroleptic-induced catatonia | - Compensation for striatal D-2 receptor deficit with "normalization" of "bottom-up modulation" | |
Acknowledgements: I thank all patients for their
participation and patience in the various studies. Without them I would
have never learned about "motor consciousness". Furthermore I am grateful
for critical discussion to all my collaborators and doctoral students as
well as to critical reviews by T.Nösselt and R.Kötter. The investigations
were financially supported by various grants from the German Research Foundation
(DFG), a Heisenberg grant, and the Novartis Foundation.
REFERENCES
Abi-Saab W, Souza D, Moghaddam B, Krystal JH (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry, 31(Suupl):104-109.
Anderson R (1999) Multimodal integration for the representation of space in the posterior parietal cortex. In: Burgess N et al. (1999), pp.90-104.
Athwal BS, Cole J, Wolpert D, Frith CD, Frackowiak R (1999) The role of proprioceptive feedback during movement: A PET study of a deafferentiated subject. Newuroimage, 9:S509.
Bechara, A., Damasio, H., Tranel, D., Damasio, AR. (1997) Deciding advantageously before knowing the advantageous strategy. Science 275:1293-95.
Baars, BJ. (1997) Some essential differences between consciousness and attention, perception, and working memory. Consciousness and Cognition 6:363-71.
Baars, B. (1998) Metaphors of consciousness and attention in the brain. Trends in Neuroscience 21(2):58-62.
Barbas H. (1995) Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev, 19(3):499-510.
Benes FM, Vincent SL, Marie A, Khan Y (1996) Up-regulation of GABA-A receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscoience, 75:1021-1031.
Binkowski F, Buccino G, Fink G, Shah NJ, Taylor JG, Seitz R (1999) Differential hemispheric activation due to visually and motor directd attention. Neuroimage, 9:S769.
Block, N. (1995) How can we find the neural correlate of consciousness? Trends in Neuroscience 19(11):456-459.
Bogerts B, Meerts E, Schönfeld-Bausch R (1985) Basal ganglia and limbic system pathology in schizophrenia. Arch Gen Psychiatry 42:784-91
Bötzel K, Ecker C, Schulze S (1997) Topography and dipole analysis of reafferent electrical brain activity following Bereitschaftspotential. Exp Brain Research, 114:352-361.
Büchel C, Friston KJ (1997) Modulation of connectivity in visual pathways by attention: Cortical interactions evaluated with Structural equation modeling and fMRI. Cerebral Cortex, 7(8):768-778.
Burgess N, Jeffery KJ, Keefe JO (Eds)(1999) The hippocampal and parietal foundations of spatial cognition. Oxford University Press, Oxford.
Bush, G, Fink, M, Petrides, G, Dowling, F, Francis, A (1996) Catatonia. Rating Scale and Standardized examination. Acta Psychiatrica Scandinavica, 93:129-143.
Carmichael ST and Price JL (1994) Architectonic subdivision of the orbital and medial prefrontal cortex in the macaquae monkey. J of Comp Neurology, 346:366-402.
Caroll B (2000) The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrum, 5(7):26-33
Castelli F, Happe F, Frith U, Frith C (2000) Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage 12:314-25.
Castiello U (1999) Mechanisms of selection for the control of hand action. Trends in Cognitive Sciences, 3:264-271.
Castiello, U., Paulignan, Y., Jeannerod, M. (1991) Temporal dissociation of motor responses and subjective awareness. A study in normal subjects. Brain 114:2639-55.
Cavada C, Goldman-Rakic P (1989) Posterior parietal cortex in Rhesus monkey: II. Evidence for segregated corticocortical networks lonking sensory and limbic areas with the frontal lobe. The J of Comp Neurology, 287:422-445.
Chalmers, D. (1996) The conscious mind. Oxford: Oxford University Press.
Chalmers, D. (1998) The problems of consciousness. In: Jasper, H. et al. (Eds) Consioucness at the frontiers of neuroscience. Advances in Neurology 77:7-19.
Colby CL. (1999) Parietal constructs action-oriented spatial representations. In: Burgess N et al. (1999), pp.104-127.
Colby CL, Duhamel JR (1996) Spatial representation for action in human cortex. Cognitive Brain Research, 5:105-115.
Coplan JD, Lydiard B (1998) Brain circuits in panic disorder. Biol Psychiatry 44:1264-76
Corbetta, M., Shulman, GL., Miezin, FM., Petersen, SE. (1993) A PET study of visuospatial attention. J Neuroscience 13:1202-26.
Crick, F. (1994) The astonishing hypothesis. Scribner's, New York.
Crick, F., Koch, C. (1998) Consciousness and Neuroscience. Cerebral Cortex 8:97-107.
Damasio AR. (1997) Towards a neuropathology of emotion and mood. Nature, 386:769-770.
Daprati E, Franck N, Georgieff N, Proust J, Pacherie E, Dalery J, Jeannerod M (1997) Looking for the agent: an investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition, 65:71-86.
Deecke L (1996) Planing, preparation, execution, and imagery of volitional action. Cognitive Brain Research 3:59-64.
DeJong H, Baruk H (1930) La catatonie experimentale par la bulbocapnine. University Press, Paris
Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST (1999) Role of posterior parietal cortex in updating reaching movements to a visual target. Nature neuroscience, 2:563-567.
Dettmers C, Fink G, Lemon R, Stephan KM, Passingham RE, Silberzweig D, Ridding M, Brooks D, Frackowiak RJ (1995) relation between cerebral activity and force in the motor areas of the human brain. J of Neurophysiology 74(2):802-15.
Devinsky, O. (1997) Neurological aspects of the conscious and unconscious mind. Annals New York Acad of Science 735:321-9.
Dias R, Robbins T, Roberts AC(1996) Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380:69-72.
Dias R, Robbins TW, Roberts AC. (1997) Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sorting test: Restriction to novel situations and independence from "on-line" processing. J of Neuroscience, 17(23):9285-97.
Dick J, Cantello R, Benecke R, Rothwell J, Marsden CD (1987) The Beretischaftspotential, L-Dopa, and Parkinsonís disease. ElectroClin Neurophysiol 66(3):263-74
Dick J, Rothwell J, Benecke R, Thompson P, Day BL, Marsden CD (1989) The Bereitschaftspotential is abnormal in Parkinsonís disease. Brain 112:233-44
Drevets WC and Raichle ME. (1998) Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implication for interactions between emotion and cognition. Cognition and Emotion, 12(3):353-385.
Driver J and Mattingley JB. (1998) Parietal neglect and visual awareness. Nature Neuroscience, 1(1):17-22.
Ebert D, Feistel H, Kaschka W (1992) Left temporal hypoperfusion in catatonic syndrome. Psychiatry Research Neuroimaging 45:239-41
Edelman, G. (1989) The remembered present: a biological theory of consciousness. Basic Books, New York.
Edelman G, Tononi G (2000 A universe of consciousness. Basic Books, New York
Feinberg, T. (1997) The irreducible perspectives of consciousness. Seminars in Neurology 17(2):85-95.
Ferrara S, Zancaner S, Orlando R, Palatini P (1999) Effects of a single dose of gaba-acid and lorazepam on psychomotor performance and subjective feelings in healthy volunteers. Eur J Clin Pharmacol 54:821-27
Fink G, Marshall J, Halligan P, Frith C, Driver J, Frackowiak R, Dolan RJ (1999) The neural consequences of conflict between intention and senses. Brain, in press.
Fink M, Bush G, Francis A. (1993) Catatonia: A treatable disorder occasionally recognized. Directions in Psychiatry, 13:1-7.
Flohr, H. (1995) Sensations and brain processes. Behavioral Brain Research 71:157-161.
Forman S, Silva A, Koretsky A (1998) Simultaneous glutamate and perfusion FMRI responses to regional brain stiimulation. J Cereb Blood Flow Metabolism 18:1064-70
Fricchione G (1985) Neuroleptic catatonia and itss relationship to psychogenic catatonia. Biol Psychiatry 20:304-313
Fricchione G, Mann S, Caroff S (2000) Catatonia, lethal catatonia, and neuroleptic malignant syndrome. Psychiatric Annals 30(5):347-355
Frith CD. (1992) The cognitive neuropsychology of schizophrenia. Lawrence Erlbaum Publishers, Hove/Sussex
Frith CD, Perry R, Lumer E (1999) The neural correlates of conscious experience: an experimental framework. Trends in Cognitive Sciences, 3(3):105-114.
Fukutake T, Hirayama K, Komatsu T. (1993) Transient unilateral catalepsy and right parietal damage. The Japanese Journal of Psychiatry, 47:647-50.
Galati G, Lobel E, Berthoz A, Pizzamiglio L, Bihan D, Vallar G (1999) Egocentric and allocentric coding of space in the human brain. Neuroimage, 9(6):S745.
Galynker I, Weiss J, Finestone H (1997) ECT treatment and cerebral perfusion in catatonia. J of Nuclear Medicine 38:251-4
Garcia C, Philpoot P, Rime B, Blin O (1997) Effects of lorazam on film-induced differentiated emotions in healthy controls. Fundam Clin Pharmacol 11:466-75
Gelenberg A.J.(1976) The catatonic syndrome. Lancet, 2:1339-1341.
Gitelmann D, Alpert N, Kosslyn S, Daffner K, Scinto L, Thompson W, Mesulam M. (1996) Functional imaging of human right hemispheric activation for exploratory movements. Ann Neurol, 39:174-9.
Gjessing R (1974) A review of periodic catatonia. Biol Psychiatry 8:23-45
Gorman J, Kent J, Coplan J (2000) Neuroanatomical hypothesis and panic disorder. Am J of Psychiatry 157:493-505
Grafton S, Hazeltine E, Ivry R (1995) Functional mapping of sequence learning in normal humans. J of Cognitive Neuroscience 7(4):497-510.
Gray JL (1995) The contents of consciousness: a neuropsychological conjecture. Behavioral and Brain Sciences
Hikosaka O, Tanaka M, Iwamura Y (1985) Deficits in manipulative behavior induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Research 325:375-80
Homburger A (1932) Motorik. In: O.Bumke (Ed) Handbuch der Geisteskrankheiten. V,9,211-64.
Horwitz B, Tagamets MA, McIntosh AR. (1999) Neural modeling, functional brain imaging, and cognition. Trends in Cognitive Sciences 3(3):91-98.
Hurley, SL. (1998) Consiousness in Action. Cambridge, Mass.: Harvard University Press.
Jahanshahi M, Jenkins ICH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. (1995) Self-initiated versus externally triggered movements. Brain, 118:913-33.
Jahanshi M and Frith C (1998) Willed action and ist implications. Cognitive Neuropsychology 15(6/7/8):483-533.
Jasper H, Descarries L, Castelluci V, Rossignol S (Eds) (1998) Consciousness at the frontiers of neurosciences. Advances in Neurology, Vol. 77, Lippincott Ė Raven, Philadelphia.
Jeannerod, M. (1997) The cognitive Neuroscience of Action. Basil Blackwell, Oxford.
Jueptner M, Stephan KM, Frith CD, Brooks D, Frackowiak R, Passingham RE. (1997) Anatomy of motor learning. Frontal cortex and attention to action. J Neurophysiology, 77:1313-24.
Kalaska J. (1996) Parietal cortex area 5 and visuo-motor behavior. Can J Physiol Pharmacol, 74:483-98.
Karnath O (1999) Spatial orientation and the representation of space with parietal lobe lesion. In: Burgess N et al. (1999), pp.50-67.
Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M (1998) The time course of brain activation during response inhibition: evidence from event-related potentials in a go/no-go task. NeuroReport 9:765-70.
Kötter R, Northoff G (2000) Medial and lateral networks in prefrontal cortex characterized by functional magnetic resonance imaging and network indices. submitted
Kraepelin, E (1927) Compendium der Psychiatrie. 9th edition, Barth Publisher, Leipzig.
Kubota K (1996) Motor cortical muscimol injection disrupts forlimb movement in freely moving monkeys. NeuroReport 7:2379-84
Kurata K, Hoffmann D (1994) Differential effects of muscimol microinjection into doral and ventral aspects of the premotor cortex of monkeys. J of Neurophysiology 71:1151-64
Lane, RD, Schwartz GE. (1987) Levels of emotional awareness: A cognitive-developmental theory and its application to psychopathology. Am J of Psychiatry 144:133-43.
Lane, RD, Ahern, GL, Schwartz, GE, Kaszniak, AW. (1997) Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry 42:834-44.
Lane, RD, Reiman, EM., Ahern, G., Schwartz, GE., Davidson, RJ. (1997) Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154:926-33.
Leary DO, Andreasen N, Luck S, Watkins G, Hichwa R (1999) The components of spatial working memory. Neuroimage, 9:S961.
LeDoux (1996) The emotional brain. Touchstone, New York
Leschinger A, Baumgart F, Bürger E, Richter A, Scheich H, Bogerts B, Northoff G (2000) Orbitofrontal cortical dysfunction and behavioral anomalies in catatonia: Auditory working memory in FMRI. Psychiatry Research Neuroimaging, in press
Levy R and Goldman-Rakic P (1999) Association of storage and processing funcion in the dorsolateral prefrontal cortex of the nonhuman primate. J of Neuroscience, 19:5149-58.
Libet, B., Wright, E., Gleason, CA., Pearl, DK. (1983) Time of conscious intention to act in relation to onset of cerebral activities (readiness potential); the unconscious initiation of a freely voluntary act. Brain 106:623-42.
Libet, B. (1985) Unconscious cereebral initiative and the role of cosncious will in voluntary action. Behavioral and Brain Sciences 8:529-66.
Libet, B. (1993) The neural time factor in conscious and unconscious events. In: Ciba Foundation: Experimental and theoretical studies of consciousness. Wiley, New York, p.123-46.
Liddle P (1994): Volition and schizophrenia. In: A.David (Ed) Neuropsychology of schizophrenia. Erlbaum, New York
Loizzo A, Scotti C, Longo V (1971) Studies on the central effects of Bulbocapnine. Psychopharmacologia 22:234-249
Luchins D, Metz R, Marks R, Cooper M (1989) Basal ganglia regional metabolism asymmetry during a catatonic episode. Biol Psychiatry 28:177
Luria A (1966) Higher cortical functions in man. Tavistock, London
MacIntosh A, Rajah N, Lobaugh N (1999) Interactions of prefrontal cortex in relation to awareness in sensory learning. Science, 284:1531-33.
Mann SC, Caroff S, Fricchione G, Campbell C (2000) Central dopamine hypoactivity and the pathogenesis of neuroleptic malignant syndrome. Psychiatric Annals 30(5):363-74
Mastermann D, Cummings J (1997) Frontal-subcortical circuits. J of Psychopharmacology 11:107-114
Mathew E, Andreason P, Pettigrww K, Carson R, King C, Paul S (1995) Benzodiazepine receptors mediate r-CBF changes in the living human brain. Proc Natl Acad Science 92:2775-9
Mattingley J, Husain M, Rorden C, Kennard C, Driver J (1998) Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature, 392:179-182.
Mayberg H, Liotti M, Brannan S, McGinnis S, Mahurin R, Jerabek P, Silva J, Tekell J, Martin C, Lancaster J, Fox PT (1999) Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J of Psychiatry, 156(5):675-682.
Merello M, Nouzeilles M, Cammarota A (1999) Effect of memantine (NMDA-antagonist) in Parkinsonís disease. Clin Neuropharmacol 22:273-6
Metzinger, T. (ED) (1995) Conscious Experience. Thorverton: Imprint Academic & Paderborn: Schöningh Press.
Meyer-Lindenberg A, Ellmore T, Brown T, Mattay V, Weinberger D, Berman K (1999) The neurophysiological substrate of volition: an event-related FMRI study. Neuroimage, 9:S364.
Miall RC and Wolpert DM (1996) Forward models for physiological motor control. Neural networks 9(8):1265-1279.
Morecraft RJ, Geula C, Mesulam M. (1992) Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J of Comp Neurology, 323:341-358.
Morecraft RJ, Hoesens G (1998) Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull, 45:209-232.
Nagel, T. (1974) What it is like to be a bat? Philosophical Review 4:435-50.
Nobre, AC., Sebestyen, GN., Gitelman, D., Mesulam, M., Frackowiak, R., Frith, CD. (1997) Functional localization of the sytsem for visuo-spatial attention using PET. Brain 120:515-33.
Nobre A, Coull J, Frith C, Mesulam MM (1999) Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nature Neuroscience 2(1):11-2.
Northoff, G. (1995) Neuropsychiatrische Phänomene und das Leib-Seele Problem. Qualia im Knotenpunkt zwischen Leib und Seele. Essen: Blaue Eule Verlag.
Northoff, G. (1995) Qualia im Knotenpunkt zwischen Leib und Seele: Argumentatives Dilemma in der gegenwärtigen Diskussion über die Subjektivität mentaler Zustände. Journal for General Philosophy of Science 26 : 269-295.
Northoff G, Wenke J, Krill W, Pflug B. (1995) Ball-experiments in 32 acute akinetic catatonic patients: Deficits of internal initiation and generation of movements. Movement Disorders, 10:589-595.
Northoff G, Wenke J, Demisch L, Pflug B. (1995) Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology, 122: 82-186.
Northoff G. (1997) Katatonie. Einführung in die Phänomenologie, Klinik und Pathophysiologie eines psychomotorischen Syndroms. Enke, Stuttgart.
Northoff, G. (ED) (1997) Neuropsychiatrie und Neurophilosophie. Paderborn: Schöningh Press.
Northoff G, Eckert J, Fritze J.(1997) Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiat, 62:404-6.
Northoff, G., Krill, W, Gille, B., Russ, M., Eckert, J., Bogerts, B., Pflug, B. (1998) Major differences in subjective experience of akinetic states in catatonic and parkinsonian patients. Cognitive Neuropsychiatry 3(3):161-78.
Northoff, G. (1999) Psychomotor phenomena as paradigmatic examples of functional brain organisation and mind-brain relationship: Do we need a "philosophy of the brain"? Philosophy, Psychology, and Psychiatry, 6,3,199-236
Northoff G, Nagel D, Danos P, Lerche J, Leschinger A, Bogerts B (1999): Impairment in visual-spatial function in catatonia: A neuropsychological investigation. Schizophrenia Research, 37:133-147.
Northoff G, Braus D, Russ M, Eckert J, Khoram-Sefat K, Leschinger A, Pflug B, Henn FA. (1999) Alterations in laterality during motor activation in FMRI in catatonia. Psychological Medicine, 29:997-1002.
Northoff G, Richter A, Gessner M, Baumgart F, Leschinger A, Danos P, Tempelmann C, Kötter R, Stepan K, Hagner T, Bogerts B, Scheich H, Heinze HJ (2000): Functional dissociation between medial and lateral orbitofrontal cortical activation pattern during negative and positive emotional stimulation: A combined FMRI/MEG study. Cerebral Cortex, 10:93-107.
Northoff G, Richter A, Gessner M, Baumgart F, Leschinger A, Danos P, Tempelmann C, Kötter R, Stepan K, Hagner T, Bogerts B, Scheich H, Heinze HJ (2000): Alteration in orbitofrontal and prefrontal cortical spatiotemporal activation pattern in catatonia during negative emotional stimulation: A combined FMRI/MEG study. in press.
Northoff A, Pfennig A, Krug M;, Schwartz A, Danos P, Bogerts B (2000) Delayed onset of late movement-related cortical potentials and abnormal response to lorazepam in catatonia. Schizophrenia Research, 44(3):193-211
Northoff G, Steinke R, Nagel D, Czerwenka C, Danos P, Krause P, Kropf S, Otto HJ, Bogerts B (1999) Right parietal cortical dysfunction in akinetic catatonia: A combined study of neuropsychology and regional cerebral blood flow. Psychological Medicine, 30:583-96
Northoff G, Pfennig A, Krug M, Bargel B, Leschinger A, Bogerts B (2000) Termination of movements and right parietal cortical dysfunction in catatonia: A MRCP study. in press.
Northoff G, Pfennig A, Krug M, Bargel B, Leschinger A, Bogerts B (2000) Termination of movements and parietal cortex: An investigation of movement-related cortical potentials. submitted
Northoff G, Böker H, Richter A, Baumgart F, Leschinger A, Lempa G, Scheich H, Heinze HJ, Bogerts B (2000) Psychological constructs of personality and orbitofrontal cortical dysfunction in catatonia: A combined study of personal constructs and FMRI. submitted
Northoff G, Richter A, Gessner M, Baumgart F, Leschinger A, Danos P, Tempelmann C, Kötter R, Stephan K, Hagner T, Bogerts B, Scheich H, Heinze HJ (1999): Gaba-ergic regulation of orbitofrontal spatiotemporal activation pattern during emotional stimulation: A combined FMRI/MEG study with placebo and lorazepam. submitted
Olney JW and Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry, 52:998-1007.
Petrides M (1995) Functional organization of the human frontal cortex for mnemonic processing. Ann New York Acad Science 769:85-96.
Petrides G, Divadeenam K, Bush G, Francis A. (1997) Synergism of lorazepam and electroconvulsive therapy in the treatment of catatonia. Biol Psychiat, 42:375-381.
Pfennig A, Krug M, Schwartz A, Leschinger A, Danos P, Bogerts B, Northoff G (1999) Termination of movements and gaba-ergic modulation by lorazepam in right parietal cortex in catatonia: A MRCP study. submitted
Posner, M. 1994. Attention: The mechanisms of consciousness. Proc Natl Acad Science 91:7398-7403.
Quintana J, Fuster J., Yajeya J (1989) Effects of cooling parietal cortex on prefrontal units in delay tasks. Brain Research, 503:100-110.
Quintana J and Fuster J (1999) From perception to action: temporal integrative functions of prefrontal and parietal neuirons. Cerebral Cortex, 9:213-221.
Richter A, Baumgart F, Leschinger A, Bogerts B, Scheich H, Northoff G (2000) Abnormal response to lorazepam in orbitofrontal cortex during emotional stimulation in catatonia: A FMRI study. submitted
Rogers D (1985) The motor disorders of severe psychiatric illness: A conflict of paradigms. Brit J Psychiatry, 147:221-231.
Rogers D (1992) Motor disorders in psychiatry. Wiley, Chichester.
Roland P, Skinhoj E, Larsen B (1980) Different coritcal areas in man in organization of voluntary movements in extrapersonal space. J of Neurophysiology 43(1):137-150.
Rolls E. (1998) The orbitofrontal cortex. In: Roberts AC, Robbins T, Weiskrantz L, (Eds) The prefrontal cortex. Oxford Unviersity Press, Oxford.
Rosebush P, Furlong B, Mazurek M. (1990) Catatonic syndrome in a general psychiatric inpatient population: Frequency, clinical presentation an response to lorazepam. J Clin Psychiatry, 51:357-362.
Sacks O (1989) Neuropsychiatry and Touretteís. In: J.Müller (Ed) Neurology and Psychiatry: A meeting of minds. Karger, New York
Satoh K, Suzuki T, Narita M, Kato T, Ohnishis H, Morita R. (1993) Regional cerebral blood flow in catatonic schizophrenia. Psychiatry Research: Neuroimaging, 50:203-216.
Saver JL, Greenstein P, Ronthal M, Mesulam M. (1993) Asymmetric catalepsy after right hemispheric stroke. Movement Disorders, 8:69-73.
Schilder P (1925) Entwurf zu einer Psychiatrie auf psychoanalytischer Grundlage. University Press, Leipzig
Searle, JR. (1998) How to study consciousness scientifically? Brain Research Reviews 26:379-87.
Selemon LD, Goldman-Rakic P (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the Rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. The Journal of Neuroscience, 8(11):4049-4068.
Siewert, CP. (1998) The significance of consciousness. Princeton: Princeton University Press.
Shin J, Kim K, Lee M (1998) Inhibitory effects of bulbocapnine on dopamine biosynthesis in PC 12 cells. Neuroscience Letters 244(3):161-4
Shore AN (1996) The experience-dependent maturation of a regulatory system in the orbital prefrontal cortex and the origin of developmental psychopathology. Development and Psychopathology 8:59-87.
Shulman G, Corbetta M, Buckner R, Raichle M, Fiez J, Miezin F, Petersen S (1997) Top-down modulation of early sensory cortex. Cerebral Cortex 7:193-206
Snowdon, JS., Crauford, D., Griffith, HL., Neary, D. (1998) Awareness of involuntary movements in Huntington disease. Arch Neurol 55:801-5.
Snyder LH, Batista AP, Anderson RA (1997) Coding of intention in the osterior parietal cortex. Nature, 386:167-170.
Spanaki MV, Siegel H, Dean A, Theodore W (1999) The effect of vigabatrin on cerebral blood flow and metabolism. Neurology 53:1518-22
Starkstein S, Petraca G, Teson A, Leiguarda R (1996) Catatonia in depression. J of Neurol, Neurosurg Psychiatry 60:326-332
Stephan KM, Thaut MH, Wunderlich G, Schicks W, Schmitz T, McIntosh A, Seitz R, Hömberg V (1999) Different awareness levels of sensorimotor processing involve distinct anatomical areas within prefrontal cortex. Neuroimage, 9:S401.
Stevens J, Wilson K, Foote W (1974) GABA-blockade, dopamine and schizophrenia: Experimental studies in the cat. Psychophamracologia 39:105-119
Stille G, Sayers A (1975) Die Immobilisationsreaktion der Ratte als tierexperimentelles Modell der Katatonie. Pharmacopsychiatry 8:105-114
Strik WK, Fallgatter AJ, Brandeis B, Pascal-Marqui RD (1999) Three dimensional topography of event-related potentials during response inhibition. Evidence for phasic frontal lo´be activation. Electrophencephalography Clin Neurophysiol, in press.
Stoerig, P. (1996) Varieties of vision: from blind responses to conscious recognition. Trends in Neurosciences 19:401-6.
Stoerig, P., Cowey, A. (1997) Blindsight in man and monkey. Brain 120:535-59.
Taylor, M.A.(1990) Catatonia. A review of the behavioral neurologic syndrome. Neuropsychiat Neuropsychol Behav Neurol, 3: 48-72.
Tononi, G, Edelman, G. (1998) Consciousness and the integration of information in the brain. In: Jaspers et al. (Eds), p.245-281.
Vallar G (1999) Spatial frames of reference and somatosensory processing: A neuropsychological perspective. In: Burgess N et al. (1999), pp.33-50.
Velmans M (1998) The relation of consciousness to the material world. In: Shear J (Ed) Explaining consciousness Ė the āhard problemĎ. p.325-337, MIT Press, Cambridge/Ma
Wang G, Volkow N, Overall J, Pascani K, Fowler J (1996) Reproducibility of regional brain metabolic response to lorazepam. J Nucl Medicine 37:1609-13
Zald D, Donndelinger M, Pardo J (1998) Elucidating dynamic brain interactions with across-subjects correlational analysis of PET data: The functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow and Metabol, 18:896-905.
Zubicaray G, zelaya F, Andrew C, Bullmore E, Williams
R, Doddrell D (1999) An fMRI study of verbal response initiation, suppression
and strategy use. Neuroimage, 9:S382.