William H. Calvin University of Washington Box 351800 Seattle WA 98195-1800 USA Email || Home Page || publication list |
as it appeared in copyright ©1974 by authors and publisher. |
Calvin, W. H. (1974). Three modes of repetitive firing and the role of threshold time course between spikes. Brain Research 69: 341-346. copyright ©1974 by authors and publisher.
William H. Calvin University of Washington Box 351800 Seattle WA 98195-1800 USA Email || Home Page || publication list |
as it appeared in copyright ©1974 by authors and publisher. |
Brain Research, 69 (1974) 341-346
Elsevier Scientific Publishing Company, Amsterdam -Printed in The Netherlands Three modes of repetitive firing and the role of threshold time course between spikesWILLIAM H. CALVIN
Department of Neurological Surgery, University of Washington School of Medicine, Seattle, Wash. 98195 (U.S.A.)
(Accepted December 13th, 1973)
When rhythmic firing is elicited in cat spinal motoneurons by long-lasting depolarizing currents, the 'threshold' voltage from which each spike arises may appear to be quite constant (Fig. 1A, arrow). If one probes for the threshold during the interspike interval (ISI), however, it is seen to fall well below this level, rising towards it later in the ISI. Exceptionally large depolarizing afterpotentials may intersect this threshold time course shortly after a spike, causing an extra spike. The extra spike itself may also similarly produce another extra spike; thus a regenerative cycle may produce a burst of spikes at a high firing rate.
Rhythmic firing was produced by steps of current injected through the intracellular recording microelectrode. Large compound excitatory postsynaptic potentials (EPSPs) could be produced by electrical stimulation of dorsal roots. The EPSPs were timed to ap pear at various points during the rhythmic firing sequence. Sixteen cats were lightly anesthetized with pentobarbital and paralyzed with gallamine; standard acute recording methods were utilized. By quantifying dV/dt near threshold, a threshold criterion was developed which corresponded to visual judgments5. All data refer to adapted firing rates within the primary range of the frequency-current relationl6; membrane potential trajectories between spikes were the scoop-ramp sequence characteristics of the primary range.
Fig. 1 illustrates 3 major properties of threshold behavior during rhythmic firing. (1) Threshold recovers rapidly after a rhythmic spike, falling below the level of the 'normal' rhythmic spike threshold (cf., constant firing level in Fig. 1A) by the time of the peak of the delayed depolarization (Fig. 1C and D). Threshold then continues to fall below 'normal' values early in the ISI. (2) Threshold rises during most of the ISI; its time course, F(t), typically intersects the membrane potential trajectory, V(t), at the end of the ISI as expected (Fig. 1B). (3) In other motoneurons, F(t) seems to intersect V(t) well before the end of the ISI as seen in Fig. ID (open arrow). After this intersection, any rapid change in membrane potential seems to elicit a sp ike. There is, however, a ceiling upon the 'accommodation' corresponding to the apparently constant thresholds of Fig. 1A; this suggests an analogy to the current ceilings described in classical accommodation experiments2. This anomalous behavior of thres hold is presumably related to phenomena seen when conditions for rhythmic firing are marginal3. Thus, while spike generation often may be described as originating from 'threshold crossing' and while one may thus characterize a 'threshold time course', the re are also situations during rhythmic firing where this descriptive characterization is inadequate.
Fig. 1. A: rhythmic firing of a cat spinal motoneuron in response to a 14 nA step of injected current through the recording Microelectrode. Delayed depolarizations following spikes are seen to 'adapt' by becoming less elevated with successive spikes. Arr ow denotes apparently constant threshold level. B: membrane potential trajectory between spikes, V(t), in another motoneuron; many adapted ISls have been superimposed photographically5. An EPSP is used to probe for threshold during the ISI; only the risin g part of the EPSP is seen, with the interpolated spike and its aftermath suppressed for clarity. The time course of the threshold, F(t), is indicated in small arrows. C, D: same motoneuron as in A. F(t) and V(t) at two different firing rates, caused by 2 5 and 14 nA currents respectively. F(t) seems to intersect V(t) before the end of the ISI (at level indicated by open arrow), unlike the more typical behavior seen in B. In attempting to relate the F(t) behavior described here to traditional notions of threshold, one must remember that rhythmic spikes are a repeated response to sustained depolarizing currents; traditional concepts of threshold and spike generation assume a silent cell and a sudden transient depolarization. Adrian1 tried to extrapolate the latter notions to explain rhythmic firing, suggesting that the relative refractory period controlled the firing rate. The alterations in the membrane potential trajector y between spikes primarily account for the changes in rhythmic firing rate24 25, not Adrian's model nor the threshold behavior described here. The rapid fall of threshold reported here may, however, account for the extra spikes which appear to arise out o f large depolarizing afterpotentials.
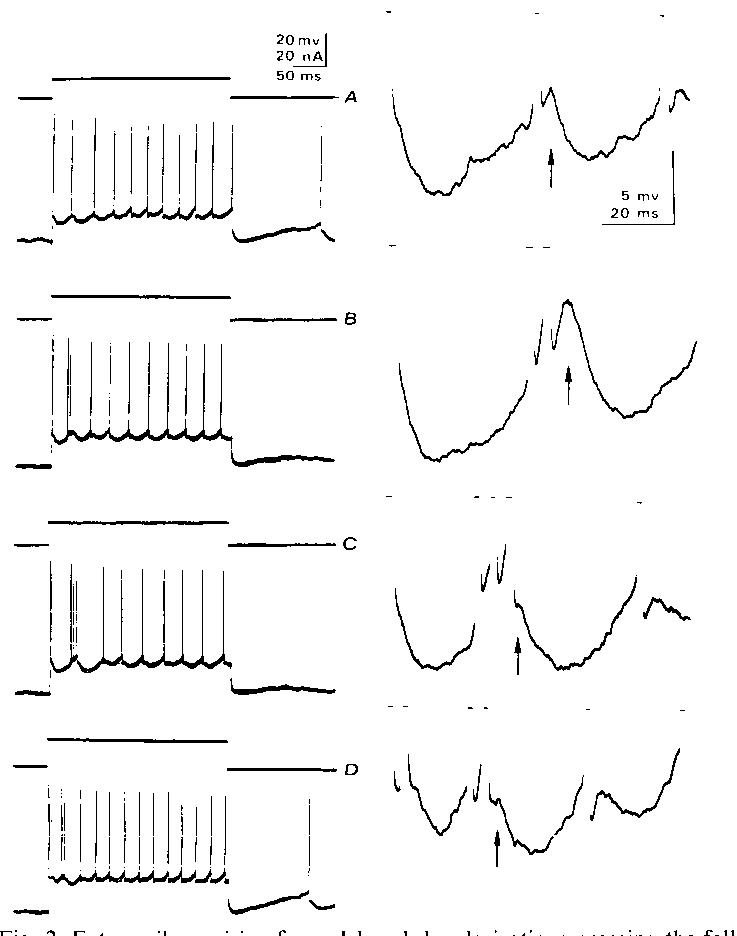
Fig. 2. Extra spikes arising from delayed depolarizations crossing the falling threshold. All records from a single motoneuron. A: trial where no extra spikes occurred; note large hump-type delayed depolarizations starting with second spike but declining with later spikes. Right column are computer magnified versions5 of the early portions of the spike trains in the left column. B: trial in which one extra spike is seen after second rhythmic spike. Note large delayed depolarization following extra spike ( arrow). C: two extra spikes are seen following second rhythmic spike. Note smaller delayed depolarization after second extra spike (arrow). D: extra spikes after both first and second rhythmic spikes. Threshold-straddling behavior seen by comparing A, B. and C, together with recovered F(t) values by the time where delayed depolarization peaks occur (Fig. IC and D), suggests that delayed depolarizations cause a regenerative cycle to produce burst firing.
Supported by grants fram the National Institutes of Health NS 09677 and NS 04053.
1 ADRIAN, E. D., The Basis of Sensation, Christophers, London, 1928, 122 pp.
2 BURKE, R. E., AND NELSON, P. G., Accommodation to current ramps in motoneurons of fast and slow twitch motor units. Int. J. Neurosci., I (1971) 347-356.
3 CALVIN, W. H., A role of motoneuron membrane potential fluctuations in the conversion of depolarization into firing frequency, XXlV Int. Congr. Physiol. Sci., Proc., 7 (1968) 212.
4 CALVIN, W. H., Synaptic potential summation and repetitive firing mechanisms: input-output theory for the recruitment of neurons into epileptic bursting firing patterns, Brain Research, 39 (1972) 71-94.
5 CALVIN, W. H., Computer-based 'kymograph' for photographing raw neurophysiological data, J. appl. Physiol., 34 (1973) 133-135.
6 CALVIN, W. H., OJEMANN, G. A., AND WARD, JR., A. A., Human cortical neurons in epileptogenic foci: comparison of interictal firing patterns to those of 'epileptic' neurons in animals, Electroenceph. clin. Neurophysiol., 34 (1973) 337-351.
7 CALVIN, W. H., AND SCHWINDT, P. C., Steps in production of motoneuron spikes during rhythmic firing, J. Neurophysiol., 35 (1972) 297-310.
8 CALVIN, W. H., AND STEVENS, C. F., Synaptic noise and other sources of randomness in motoneuron interspike intervals, J. Neurophysiol., 31 (1968) 574-587.
9 CALVIN, W. H., SYPERT, G. W., AND WARD, JR., A. A., Structured timing patterns within bursts from epileptic neurons in undrugged monkey cortex, Exp. Neurol., 21 (1968) 535-549.
9a DENSLOW, J. S., Double discharges in human motor units, J. Neurophysiol., 11 (1948) 209-215.
10 ECCLES, J. C., AND HOFF, H. E., The rhythmic discharge of motoneurons, Proc. roy. Soc. B. 110 (1932) 483-514.
11 EYZAGUIRRE, C., AND KUFFLER, S. W., Further study of some, dendrite, and axon excitation in single neurons, J. gen. Physiol., 39 (1955) 121-153.
12 GELFAN, S., KAO, G., AND LING, H., The dendritic tree of spinal neurons in dogs with experimental hindlimb rigidity, J. comp. Neurol., 146 (1972) 143-174.
GRAMPP, W., Firing with multiple-spike discharges in the slowly adapting stretch receptor neuron of the lobster, Acta physiol. scand., 66 (1966) 484 494.
13a HOFF, H. E., AND GRANT, R. S., The supernormal period in the recovery cycle of motoneurons, J. Neurophysiol., 7 (1944) 305-322.
14 KANDEL, E. R., AND SPENCER, W. A., Electrophysiology of hippocampal neurons. II. Afterpotentials and repetitive firing, J. Neurophysiol., 24 (1961) 243-259.
15 KERNELL, D, The delayed depolarization in cat and rat motoneurons. In J. C. ECCLES AND J. P. SCHADE (Eds.), Physiology of Spinal Neurons, Progr. Brain Res., Vol. 12, Elsevier, Amsterdam, 1964, pp. 42-55.
16 KERNELL, D., The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurons stimulated by long-lasting injected currents, Acta physiol. scand., 65 (1965) 65-73.
17 KJERULF, T. D., O NEAL, J. T., CALVIN, W. H., LOESER, J. D., AND WESTRUM, L. E., Deafferentation effects in lateral cuneate nucleus of the cat: correlation of structural alterations with firing pattern changes, Exp. Neurol., 39 (1973) 86-102.
18 KOIKE, H., MANO, N., OKADO, Y., AND OSHIMA, T., Repetitive impulses generated in fast and slow pyramidal tract cells by intracellularly applied current steps, Exp. Brain Res., 11 (1970) 263-281.
19 LØMO, T., AND ROSENTHAL, J., Control of ACh sensitivity by muscle activity in the rat, J. Physiol. (Lond.), 221 (1972) 493-513.
20 NELSON, P. G., AND BURKE, R. E., Delayed depolarization in cat spinal motoneurons, Exp. NeuroL, 17 (1967) 16 26.
21 PORTER, R., Early facilitation at corticomotoneuronal synapses, J. Physiol. (Lond.), 207 (1970) 733-746.
22 PORTER, R., AND MUIR, R. B., The meaning for motoneurons of the temporal pattern of natural activity in pyramidal tract neurons of conscious monkeys, Brain Research, 34 (1971) 127-142.
23 RUTLEDGE, L. T., DUNCAN, J., AND CANT, N., Long-term status of pyramidal cell axon collaterals and apical dendritic spines in denervated cortex, Brain Research, 41 (1972) 249-262.
24 SCHWINDT, P. C., Membrane potential trajectories underlying motoneuron rhythmic firing at high rates, J. Neurophysiol., 36 (1973) 431 449.
25 SCHWINDT, P. C., AND CALVIN, W. H., Membrane potential trajectories between spikes underlying motoneuron firing rates, J. Neurophysiol., 35 (1972) 311-325.
26 WASHIZU, Y., AND TERZUOLO, C. A., Impulse activity in the crayfish stretch receptor neuron, Arch. ital. Biol., 104 (1966) 181-194.
27 WESTRUM, L. E., WHITE, JR., L. E., AND WARD, JR., A. A., Morphology of the experimental epileptic focus, J. Neurosurg., 21 (1964) 1033-1046.