Calvin, W. H., & Sypert, G. W. (1975). Cerebral cortex neurons with extra spikes: a normal
substrate for epileptic discharges? Brain Research 83: 498-503
copyright
©1975 by authors and publisher
Calvin, W. H., and Sypert, G. W., Cerebral cortex neurons with extra spikes, BRAIN RESEARCH (1975)
 Webbed Reprint Collection
Webbed Reprint Collection
William H. Calvin
University of Washington
Box 351800
Seattle WA 98195-1800 USA
Email || Home
Page || publication list
|
as it appeared in
Brain Research 83: 498-503 (1975)
copyright ©1975 by authors and publisher.
|
scanned, OCR, webbed -- but NOT proofread -- 11 Jan 97
Brain Research, 83 (1975) 498-503 Elsevier Scientific Publishing Company, Amsterdam -Printed in The Netherlands
Cerebral cortex neurons with extra spikes:
a normal substrate for epileptic discharges?
WILLIAM H. CALVIN and GEORGE W. SYPERT
Department of Neurological Surgery,
University of Washington School of Medicine,
Seattle, Wash. 98195 (U.S.A.)
(Accepted October 14th, 1974)
Double spikes during otherwise rhythmic discharge were frequently observed in 'fast' pyramidal tract neurons in response to steady depolarizing currents injected through the recording micropipette. The extra spike appears to arise from a large depolarizin
g afterpotential crossing the falling threshold several milliseconds following a spike; extra spikes themselves may generate further extra spikes in a similar manner, generating burst patterns which are strikingly similar to those of epileptic neurons.
Such conclusions are based upon an ability to identify 3 distinct modes of repetitive firings. When each action potential ('spike') in a repetitive train appears to be associated with discrete synaptic inputs which summate to cross the threshold (Fig. 1J)
, it is natural to view the neuron's input-output conversion function as analogous to an AND gate even though, in the more typical situations, the individual input components are so small that they cannot be readily distinguished in a depolarizing wave (f
or example, the first synaptic wave in Fig. IJ). When such a wave is large enough to cross the threshold occasionally, an irregular spike train is generated. The AND gate concepts which arise from such examples have traditionally furnished the prevalent n
europhysiological view of neuron input-output conversion; however, two other modes of repetitive spike generation exist which imply rather different conversions6.
Calvin6 has proposed that repetitive firing, such as seen in Fig. 1J, be denoted the 'occasional spike mode' to distinguish it from the more rhythmic discharges which arise if a longer-lasting synaptic wave should attempt to hold the membrane p
otential above the threshold. This 'rhythmic firing mode' is characterized by a rather linear6, 9, 11, 21 conversion of sustained synaptic currents into discharge rates in a manner analogous to current-controlled oscillators.
Standard intracellular recording techniques were employed, including moderate capacity neutralization. Steady currents were passed using a bridge circuit. Micropipettes were beveled3 and filled with KCI; they were selected for their ability to pass 20 nA
currents without polarization (resistance range 4-8 Mohm). Twenty cats were anesthetized with pentobarbital (moderate to deep levels) and paralyzed with gallamine9. To minimize cortical pulsations, we used wide bifrontal exposure, cisterna! drainage, a pr
esser foot, and a bilateral pneumothorax with the rib cage held in an expanded position. Most recordings were from postcruciate gyrus, area praecentralis gigantopyramidalisl6. A concentric stimulating electrode in the medullary pyramids (posterior approac
h) served to identify antidromically and classify pyramidal tract (PT) cells by conduction velocity; 'fast' are > 20 m/see, following Takahashi28. The surface electrocorticogram (ECoG) was recorded with a surface electrode near the penetration site. Becau
se formerly healthy neurons will, upon deterioration of the spike heights below about 60 mV, often exhibit only transient firing to sustained current, we have selected for this report only neurons whose spikes peaked 70-130 mV above the resting potential
and which had stable frequency-current curves.
Whether a synaptic wave (for example, the ones associated with the spindles in the surface electrocorticogram) elicits occasional or rhythmic mode discharges depends in part upon the biasing created by other inputs to the neuron. To demonstrate this, we m
imicked steady synaptic currents by injecting steady currents through the intracellular
recording Microelectrode. Spindle-associated synaptic waves27 arising from the—80 mV resting
potential occasionally crossed threshold (Fig. IJ).
If injected currents depolarized the membrane enough to set up a continuous rhythmic discharge, the spindle inputs then merely modulated the firing rate (Fig. IK). When we explored with steps of injected current during the quiescent periods between spindl
es, we found the adapted firing rate to increase linearly with current (Fig. IE) as shown by earlier workers1l 2l. In this particular neuron, as in many others, unitaryappearing events in the baseline could sometimes be seen (as in Fig. II)l It l3'l4 22 s
uggesting either unitary postsynaptic potentials from a single bursting input or repetitive firing in a dendritic spike generator.
It has been recently noted6,6a that a third mode of repetitive firing may be deduced in records from many different preparations; it typically takes the form of a 'doublet', consisting of an extra spike appearing several milliseconds following a spike. In
motoneurons6 9, in crustacean stretch receptorsl5, and in hippocampal pyramidal neuronsl8, such doublets (or triplets, etc.) are clearly associated with a large depolarizing'afterpotential' following the spike; when large enough, these humps appear to ri
se through the falling threshold several milliseconds after the spike, thus eliciting an extra
spike.
In 8 of our 'fast PT' neurons, we have observed such doublet discharges. One may find injected current strengths at which the doublets appear occasionally in the otherwise rhythmic discharge (Fig. IC); examination of the membrane potential trajectory betw
een spikes (Fig. 1 G) reveals a large hump-like depolarizing afterpotential from which the extra spike appears to arise. Higher currents cause the doublets to become more prominent (Fig. ID), unlike the doublets in spinal motoneurons which disappear when
the rhythmic firing rate is elevated6,l7.
These neurons exhibit some tendency for long interspike intervals to follow short ones, e.g., the lengthened intervals which follow the doublets in Fig. 1C. The doublets, however, do not merely represent the inverse of this relation, e.g., short tending t
o follow long. They are not only short intervals; they are stereotyped, very short intervals, such as the ones seen in the external cuneatenucleus7 20. Fig. I H shows a joint interval density, where successive intervals are plotted against each other26. O
rdinary rhythmic firing at various rates produces points along the 45° diagonal; the points clustered at about 2 msec parallel to the axes demonstrate the lack of gradation of this unique short interval phenomenon. Since 2 msec corresponds to the peak of
the hump following the rhythmic spike (Fig. 1 G), one may say that these are 'extra spikes' produced by the depolarizing aftermath of a previous spike. Sometimes a triplet is seen (Fig. 1L), suggesting that the hump following the extra spike itself also e
licits another extra spike in a regenerative cycles. This constitutes a mode of repetitive firing with distinctly different properties from the occasional and rhythmic modes.
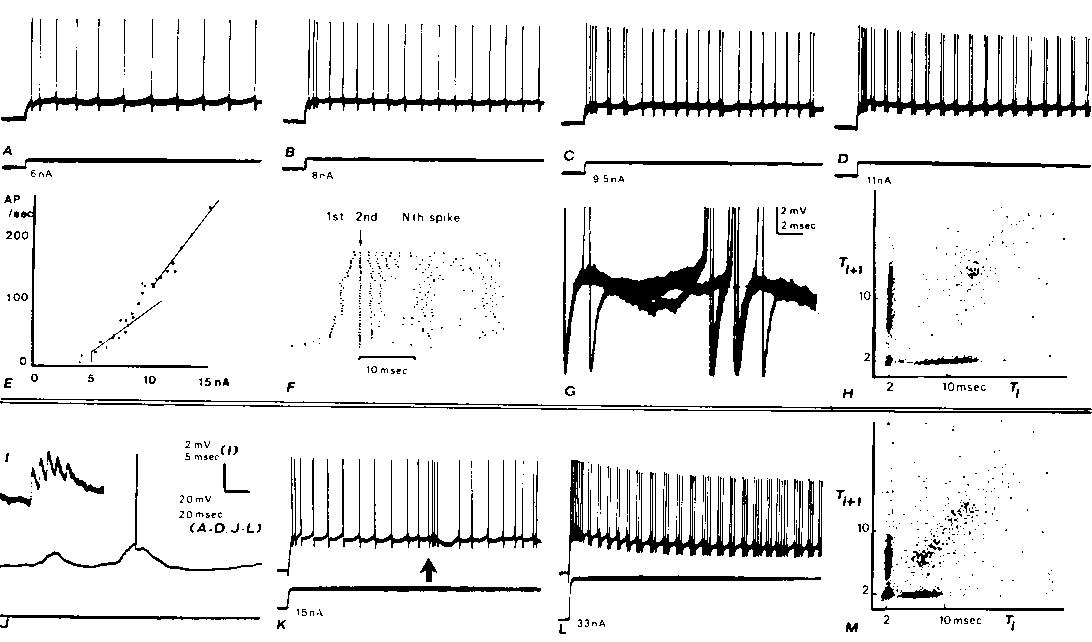
Fig. 1. Intracellular recordings from two cortical neurons (A-H, I-M) with axons projecting into the medullary pyramids with fast conduction velocities. Both neurons exhibit doublet discharges during otherwise rhythmic firing from steps of cur
rent injected through the recording micropipette (A). Rhythmic firing evoked by a 6 nA step. B: in response to 8 nA, the rhythmic firing rate increases and a 2 msec interspike interval is seen after the second rhythmic spike. C: a doublet discharge is see
n occasionally during otherwise rhythmic firing at 9.5 nA. D: with 11 nA (and greater) currents, doublet discharges recur rhythmically. E: plot of adapted firing frequency versus the injected current. At low currents before doublets are seen during adapte
d discharge, the frequency-current relation has a slope of 13.6 spikes/sec/nA. At higher currents, when doublet discharge is well-established during maintained firing, the slope is 25 spikes/sec/nA. The intermediate points which have not been fitted with
straight lines are those currents at which mixed responses were elicited (as in C). F: raster displays which aligns the second spike of each response below the arrow: dots representing the first spikes appear to the left of the arrow. Data from many diffe
rent current steps (range as in E) arranged in ascending order. The 11 nA step was repeated 12 times. G: membrane potential trajectory between spikes during adapted firing; 3 superimposed sweeps triggering upon a spike several hundred msec after the begin
ning of the train in C. In 2 cases, no doublet occurred and a hump can be seen following the spike; in the third case, an extra spike is seen arising from a level just above this hump. H: joint interval density is a scatter plot of interspike interval ver
sus the following interspike interval. Plot mixes the responses from the entrire range of current values shown in E and F. The 'arrow pointing to the origin' shape of this plot is characteristic of the fast PT neurons which develop doublets at higher rhyt
hmic rates (see M). I-M: similar records from another fast PT neuron. The effects of a large synaptic input associated with a spindle in the electrocorticogram (ECoG) are shown in I-K. I: large unitary-appearing events in synaptic noise. I: spindle associ
ated synaptic waves arising from the —80 mV resting potential elicit occasional spikes. K: low-frequency rhythmic response to a current step is interrupted by a spindle wave at arrow; note gradual rise in firing rate. L: after exhibiting a progressive dev
elopment of doublets as illustrated for the other neuron in A-D, this neuron developed triplets and even longer clusters of extra spikes at higher currents. (Baseline changes are due to tip potential alteration with current.) Thus high-frequency bursts ma
y be due to either large synaptic waves (as in K) or to an extra spike mode of rhythmic firing. M: joint interval density as in H.
Koike et al.21 have noted that fast PT cells may exhibit an unusual adaptation pattern in that the declining firing rates do not begin until after an initial rise in rate, i.e., the first interspike interval may be longer than the second in response to a
current step. In our fast PT cells exhibiting doublets, it was apparent that the adaptation pattern also involved the extra spike mode. The raster displays in Fig. IF aligns the second spike of the responses to various current steps. It is apparent that t
he stereotyped doublet interval begins to appear after the second rhythmic spike in Fig. 1B; at higher currents, higher in the raster, a triplet is seen (Fig. 1C) which suggests that the depolarizing aftermath of the extra spike has itself generated anoth
er extra spike. One may thus get a stereotyped 'burst' following the second spike of the response which is little different over wide variation in levels of input. For the fast PT cell illustrated in Fig. IKL, this long-first-interval pattern was not seen
.
In epileptogenic cerebral cortex, both humane and in chronic monkey preparationsl0'l2 29 30, many neurons observed with extracellular recording will spontaneously exhibit high-frequency burst firing patterns. While the firing patterns of many of these neu
rons are often sufficiently variable from one burst to the next as to suggest a large, variable synaptic wave driving the rhythmic firing mode to high rates2, other neurons exhibit very stereotyped burst patterns. We earlier showed10 that some of these ne
urons exhibit 'long-first-interval' patterns in which the burst may be stereotyped starting with the second spike, with the first spike standing in front at various intervals. All such neurons are fast PT cells, in the case of monkeys12. Spinal motoneuron
s not infrequently exhibit depolarizing afterpotentials which begin after the second spike of a rhythmic trains 9, making extra spikes more likely to occur after the second spike; this tendency may explain many long-first-interval phenomena (but see Wyler
et al.29).
The origin of the depolarizing humps is not well understood; in spinal motoneurons19 23, recurrent axon synaptic effects have been ruled out and dendritic currents suggested as a mechanisms.. Grampp15 has demonstrated in lobster stretch receptors using ex
tracellular field maps and simultaneous intra-dendritic and intrasomatic recording that the humps are correlated with the antidromic invasion of the dendritic tree by the spike. Thus, at the time the soma repolarizes, the remnants of the spike are still l
eft in the dendrites and cause depolarizing current to flow to the soma in a manner analogous to synaptic potentials. Due to the extensive apical and basal dendritic trees of PT neurons, this explanation is an obvious candidate for the mechanism underlyin
g the hump in PT neurons.
Judging from our present observations, the stereotyped high-frequency bursts probably arise from the extra spike mode. For this class of epileptic neurons, then, the high-frequency burst could be a property of the cell's repetitive firing mechanism and no
t necessarily associated with a massive synaptic input2; indeed, it is apparent from Fig. 1
that long-first-interval bursts can arise from input currents in the lower third of the
frequency-depolarization curve.
Burst firing patterns provide potent inputs to downstream cells5~24 25. When minor inputs can produce major outputs from cells, one suspects that such cells could play a critical role in the recruitment of other cells into the burst firing patterns which
characterize the interictal epileptogenic focus5. We thus believe that the extra spike mode may play an important role in epileptogenic cerebral cortex.
Supported by NIH Grants NS 04053, NS 05211 and NS 09677.
We thank Jerrold Maddocks and Susan Johnston for their technical assistance.
1 ATKINSON, J. R., AND WARD, A. A., JR., Intracellular studies of cortical neurons in chronic epileptogenic foci in the monkey, Exp. Neurol., 10 (1964) 285-295.
2 AYALA, G. F., DICHTER, M., GUMNIT, R. J., MATSUMOTO, H., AND SPENCER, W. A., Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms, Brain Research, 52 (1973) 1-17.
3 BARRETT, J. N., AND GRAUBARD, K., Fluorescent staining of cat motoneurons in vivo with beveled micropipettes, Brain Research, 18 (1970) 565-568.
4 CALVIN, W. H., Evaluating membrane potential and spike patterns by experimenter-controlled computer displays, Exp. Neurol., 21 (1968) 512-534.
5 CALVIN, W. H., Synaptic potential summation and repetitive firing mechanisms: input-output theory for the recruitment of neurons into epileptic bursting firing patterns, Brain Research 39 (1972) 71-94.
6 CALVIN, W. H., Three modes of repetitive firing and the role of threshold time course between spikes, Brain Research 69 (1974) 341-346.
6a CALVIN, W. H., Generation of spike trains in CNS neurons, Brain Research 84 (1975)1-22.
7 CALVIN, W. H., AND LOESER, J. D., Stereotyped doublet and burst firing patterns in neurons of normal lateral cuneate nucleus: evidence for a normal substrate for epileptic burst responses, Electroenceph. clin. Neurophysiol., (1974) (abstract, in press).
8 CALVIN, W. H., OJEMANN, G. A., AND WARD, A. A., JR., Human cortical neurons in epileptogenic foci: comparison of interictal firing patterns to those of 'epileptic' neurons in animals, Electroencephalography and Clinica
l Neurophysiology 34 (1973) 337-351.
9 CALVIN, W. H., AND SCHWINDT, P. C., Steps in production of motoneuron spikes during rhythmic firing, Journal of Neurophysiology 35 (1972) 297-310.
10 CALVIN, W. H., SYPERT, G. W., AND WARD, A. A., JR., Structured timing patterns within bursts from epileptic neurons in undrugged monkey cortex, Experimental Neurology 21 (1968) 535-549.
11 CREUTZFELDT, O. D., Lux, H. D., AND WATANABE, S., Electrophysiology of cortical nerve cells. In D. P. PURPURA AND M. D. YAHR (Eds.), The Thalamus, Columbia University Press, New York, 1966, pp. 209-235.
12 FETZ, E. E., AND WYLER, A. R., Operantly conditioned firing patterns of epileptic neurons in the monkey motor cortex, Exp. Neurol., 40 (1973) 586-607.
13 GLOTZNER, F. L., Intracellular recording of spontaneous local responses in the chronic epileptogenic focus of a rhesus monkey, Exp. Neurol., 42 (1974) 233-237.
14 GLOTZNER, F. L., FETZ, E. E., AND WARD, A. A., JR., Neuronal activity in the chronic and acute epileptogenic focus, Exp. Neurol., 42 (1974) 502-518.
15 GRAMPP,W., The impulse activity in different parts of the slowly adapting stretch receptor neuron of the lobster, Acta physiol. scand., 66, Suppl. 262 (1966) 3-36.
16 HASSLER, R., UND MUMS-CLEMENT, K., Architektonischer Aufbau des sensomotorischen und parietalen Cortex der Katze, J. Hirnforsch., 6 (1964) 377-420.
17 HOFF, H. E., AND GRANT, R. S., The supernormal period in the recovery cycle of motoneurons, J. Neurophysiol., 7 (1944) 305-322.
18 KANDEL, E. R., AND SPENCER, W. A., Electrophysiology of hippocampal neurons. II. Afterpotentials and repetitive firing, J. Neurophysiol., 24 (1961) 243-259.
19 KERNELL, D., The delayed depolarization in cat and rat motoneurones, Progr. Brain Res., 12 (1964) 42-55.
20 KJERULF, T. D., O NEAL, J. T., CALVIN, W. H., LOESER, J. D., AND WESTRUM, L. E., Deafferentation effects in lateral cuneate nucleus of the cat: correlation of structural alterations with firing pattern changes,
Experimental Neurology , 39 (1973) 86-102.
21 KOIKE, H., MANO, N., OKADA, Y., AND OSHIMA, T., Repetitive impulses generated in fast and slow pyramidal tract cells by intracellularly applied current steps, Exp. Brain Res., 11 (1970) 263-281.
22 LI, C.-L., Cortical intracellular synaptic potentials, J. cell. comp. PhysioL, 58 (1961) 153-168.
23 NELSON, P. G., AND BURKE, R. E., Delayed depolarization in cat spinal motoneurons, Exp. Neurol., 17 (1967) 16-26.
24 PORTER, R., Early facilitation at corticomotoneuronal synapses, J. Physiol. (Lond), 207 (1970) 733-746.
25 PORTER, R., AND MUIR, R. B., The meaning for motoneurones of the temporal pattern of natural activity in pyramidal tract neurones of conscious monkeys, Brain Research, 34 (1971) 127-142.
26 RODIECK, R. W., KIANG, N. Y.-S., AND GERSTEIN, G. L., Some quantitative methods for the study of spontaneous activity of single neurons, Biophys. J., 2 (1962) 351-368.
27 SPENCER, W. A., AND BROOKHART, J. M., A study of spontaneous spindle waves in sensorimotor cortex of cat, J. Neurophysiol., 24 (1961) 50-65.
28 TAKAHASHI, K., Slow and fast groups of pyramidal tract cells and their respective membrane properties, J. Neurophysiol., 28 (1965) 908-924.
29 WYLER, A. R., FETZ, E. E., AND WARD, A. A., JR., Spontaneous firing patterns of epileptic neurons in the monkey motor cortex, Exp. Neurol., 40 (1973) 567-585.
30 WYLER, A. R., FETZ, E. E., AND WARD, A. A., JR., Injury-induced long-first-interval bursts in cortical neurons, Exp. Neurol., 41 (1973) 773-776.
