Marco Iacoboni1,2, MD, Itzhak Fried3, MD, PhD, and Eran Zaidel2, PhD
Department of Neurology, UCLA School of Medicine1
Department of Psychology2
Division of Neurosurgery, UCLA School of Medicine3
University of California, Los Angeles
Key words: Corpus callosum - Interhemispheric transfer time - Partial commissurotomy
Address correspondence to:
Marco Iacoboni, MD
Reed Neurological Research Center
Department of Neurology, UCLA School of Medicine
710 Westwood Plaza
Los Angeles, California 90024-1769
Tel (310) 206-3992 Fax (310) 794-7406
e-mail: iacoboni@loni.ucla.edu
Abstract
Interhemispheric transmission time was measured in a patient before and after partial commissurotomy sparing the splenium of the corpus callosum. The transmission time was measured using a simple reaction time paradigm with unimanual responses to lateralized flashes at 4 and 8 degrees of eccentricity. Post-operative transfer time was longer than pre-operative transfer time at 8 degrees but not at 4 degrees of eccentricity. These data do not support the notion that the callosal transfer time is always faster through motor rather than visual fibers. They rather suggest that the callosal transfer time through visual fibers is longer than the callosal transfer time through motor fibers only for flashes at large eccentricities.
Introduction
Unimanual simple reaction times (RT) to lateralized flashes can be used to estimate interhemispheric transmission time (ITT) of visuo-motor information. Uncrossed responses (response hand ipsilateral to the visual stimulus) do not require interhemispheric transfer, whereas crossed responses (hand contralateral to the stimulus) require information transfer from one hemisphere (processing the visual stimulus) to the other (controlling the motor output).1 The difference between the two conditions is on average 3-4 msec2 and it is assumed to reflect transfer through the corpus callosum. Marked increases of the ITT in subjects with agenesis of the corpus callosum and even larger ones in commissurotomy patients2,3 support this assumption.
According to Berlucchi et al.4, changes in the ITT as a function of variations in visual parameters of the stimulus would indicate that interhemispheric transfer occurs through visual rather than motor channels. Variations in visual stimulus parameters do affect the ITT in callosal agenesis and in split brain3 patients. In these patients the transfer may occur via the anterior commissure connecting temporal visual association areas5, or via the posterior and intercollicular commissures connecting the pretectal nuclei and the superior colliculus.6 In normal subjects, however, variations in visual stimulus parameters such as retinal eccentricity or intensity3-4 affect RT for both crossed and uncrossed conditions but do not significantly affect the ITT. Moreover, patients suffering from a motor deficit as a result of unilateral anterior cortical lesions show a larger ITT than patients with a unilateral cortical lesion in similar areas but without motor symptoms.7 These findings suggest that the transfer normally occurs through motor rather than visual channels.
Data from studies with monkeys and humans8-9 show a topographic map of callosal fibers in relation to cortical regions of origin and termination. The rostrum and the genu interconnect prefrontal cortex. The anterior midbody interconnects premotor and supplementary motor cortex, whereas the posterior midbody relates to primary motor and somatosensory cortices. The isthmus contains superior temporal and posterior parietal fibers, whereas the splenium contains occipital and inferior temporal fibers. According to these findings, a direct answer to the question regarding the callosal channel of interhemispheric transfer during simple reaction times to lateralized flashes can be provided by studying patients with selective surgical lesions of the corpus callosum, who have both pre- and post- surgery data.
We varied the eccentricity of the visual stimulus and measured the ITT in a patient before and after partial commissurotomy sparing few fibers of the rostrum and sparing the splenium. The prediction was straightforward: if the normal callosal route is motor or pre-motor, then the eccentricity should affect ITT after but not before surgery. If visual transfer is longer than motor transfer, as suggested by visual evoked potential data10, then we would also predict longer ITT after than before surgery.
Materials and Methods
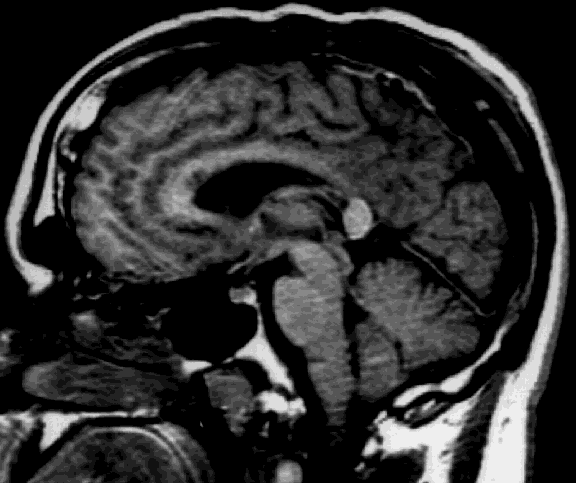
Case report. D.W. is a 30 year old right-handed man with a history of chronic seizures. His seizures started at the age of five and were characterized by loss of contact and automatism. In 1989, the patient developed a second type of seizures which he described as "drop attacks". These episodes consisted of sudden falls to the ground without any warning and resulted in numerous injuries. Despite pharmacological therapy with multiple drugs, the patient's seizures did not improve. EEG recordings showed that most seizures appeared to have a left frontal onset, but that some had a more diffuse onset. Magnetic resonance imaging and positron emission tomography showed no abnormality. The patient underwent the carotid Amytal procedure (Wada test) showing left hemisphere dominance for language. As the patient was unlikely to have a single seizure focus, he was not deemed a good candidate for resective surgery. To control the most disabling feature of his seizure disorder, namely the drop attacks, anterior corpus callosum section was performed. The callosum was divided initially at midbody and subsequently proceeding anteriorly to the genu. Dissection then proceeded posteriorly, and the section carried out to the level of the splenium (Figure 1).

Fig. 1: Sagittal view of the post-operative MRI showing that the splenium was spared. Few fibers of the rostrum were also spared and they are more easily visible in other slices.
Postoperative course was unremarkable. In particular, the patient's visual fields were normal after the surgery. The patient was discharged home after six days. During the eight months follow up since his operation, he had no "drop attacks". His partial seizures continue but are reported to be less severe.
Apparatus and procedure. The patient was informed of the nature of the study, but he was naive as to the aim of the investigation. He was seated in a dimly lit room at a distance of 57.3 cm from a high resolution RGB color monitor of a MacIntosh IIsi computer, with his chin in a chinrest and the eyes aligned with a fixation cross in the middle of the screen. Stimuli (black flashes on a grey background) were presented for 50 msec. A response panel placed at midline was used for manual responses.
In each testing session, there were eight blocks of 40 trials. Flashes appeared from 500 msec to 2500 msec after a warning tone lasting 100 msec and they were presented in four blocks at 4 degrees from the vertical meridian and in the remaining four blocks at 8 degrees from the vertical meridian, according to a counterbalanced sequence (48844884). The use of the right (R) or the left (L) hand was counterbalanced across blocks according to the following sequence: RLRLLRLR. In each block flashes were randomly presented in both visual fields (half of the trials in the left one and half in the right one). Manual response was carried out by pressing with the index finger a key placed over the panel. For each responding hand in each eccentricity the patient had a warm up session of 20 trials. The patient's eye movements were monitored during all the testing sessions by the first Author and when eye movements occurred the trial was discarded.
Data analysis. Reaction times (RTs) shorter than 150 msec were considered anticipatory errors and RTs longer than 500 msec were considered attentional errors and both were removed from the analyses. This procedure led to small differences in the number of data points per condition, which is reflected in small changes in the means of combined conditions (such as the mean of crossed conditions which combines the crossed condition at 4 and at 8 degrees of eccentricity) presented in the Results section. RTs were submitted to analyses of variance (ANOVAs) with trials as the random variable, and with eccentricity and crossed-uncrossed condition as between-trial variables. Since the patient was available only for one testing session before the surgery, limiting the number of pre-operative trials, we performed separate ANOVAs for pre- and post-operative data.
Results
Five testing session were performed, one before and four after surgery. Overall RTs of the pre-operative testing session (293.9 msec) were shorter (p<.001) than the overall RTs of the post-operative testing sessions (313.9 msec). Table 1 summarizes means, standard errors, and ITT before and after surgery at 4 and 8 degrees of eccentricity.
Table 1: Reaction times and ITT values (msec) before and after commissurotomy
4 degrees
|
8
degrees
|
ITT
| ||||
| Before
commissurotomy
|
Crossed
|
Uncrossed
|
Crossed
|
Uncrossed
|
4
degrees
|
8
degrees
|
| Mean
|
293.9
|
288.8
|
298.1
|
294.6
|
5.1
|
3.5
|
| St.
Error
|
6.8
|
5.7
|
7.4
|
6.2
|
||
| After
commissurotomy
|
||||||
| Mean
|
311.2
|
305.4
|
331.4
|
310.8
|
5.8
|
20.6
|
| St.
Error
|
2.9
|
2.9
|
3.8
|
3.3
|
||
Pre-operative testing session
A 2 (eccentricity: 4 degrees, 8 degrees) x 2 (condition: crossed, uncrossed) ANOVA on the RTs of the pre-operative testing session revealed no main effect of eccentricity, with RTs to the inner location of the stimulus (291.4 msec; st. error 4.5) not different from RTs to the outer location of the stimulus (296.3 msec; st. error 4.8), no main effect of condition, with crossed conditions (295.9 msec; st. error 5.0) not different from the uncrossed ones (291.7 msec; st. error 4.2), and no significant interaction between eccentricity and condition.
Post-operative testing sessions
A 4 (testing session: first, second, third, fourth) x 2 (eccentricity: 4 degrees, 8 degrees) x 2 (condition: crossed, uncrossed) ANOVA on the RTs of the post-operative testing sessions revealed a main effect of testing session (p>.001). This main effect was tested using an orthogonal contrast assuming a linear trend15. The linear contrast was highly significant (p>.001) suggesting a linear recovery from the first session (370.0 msec; st. error 3.4) through the second (326.0 msec; st. error 3.1), the third (290.8 msec; st. error 1.9) , and the fourth session (283.4 msec; 2.6). However, testing session did not interact with any other experimental variable. The ANOVA revealed a main effect of eccentricity (p<.001), with RTs to the inner location of the stimulus (308.3 msec; st. error 2.0) shorter than RTs to the outer location of the stimulus (321 msec; st. error 2.5), and a main effect of condition (p<.001), with crossed conditions (320.1 msec; st. error 2.4) slower than the uncrossed ones (307.8 msec; st. error 2.2).
A significant eccentricity by condition interaction was also found (p<.02). There was no difference at 4 degrees of eccentricity between uncrossed (305.4 msec; st. error 2.9) and crossed (311.2 msec; st. error 2.9) conditions, whereas there was a difference (p<.001) at 8 degrees of eccentricity between uncrossed (310.8 msec; st. error 3.3) and crossed (331.4 msec; st. error 3.8) conditions (Figure 2).
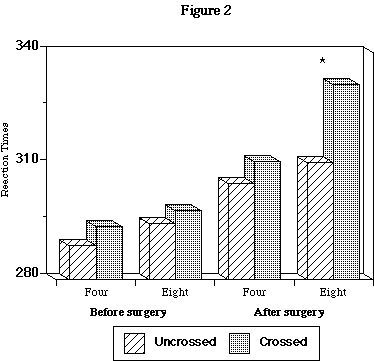
Fig. 2: Four= degrees of retinal eccentricity. Eight= degrees of retinal eccentricity. * = significant difference, p<.001
Discussion
To our knowledge, this is the first report of ITT in humans measured before and after partial commissurotomy. The overall RT of the post-operative testing sessions showed an expected slowing down compared to the pre-operative values. The recovery of overall RT up to pre-operative values was rapid (the third testing session was performed one month after the surgery) and linear, as showed by the contrast analysis. The lack of interaction between crossed-uncrossed conditions and testing sessions is a confirmation of the independence of ITT and overall RT, as previously suggested by normal subjects' data2-3. The lack of interaction between eccentricity and crossed-uncrossed conditions before the operation and its appearance after the surgery confirms our prediction and strongly suggests that the interhemispheric transfer of information usually occurs through motor fibers. Mid-sagittal view of the post-operative MRI shows that the surgical procedure spared the splenium. Thus, in D.W., after the commissurotomy the transfer can take place at the level of the splenium. Consequently, our data are in line with the prediction of Berlucchi et al.4 that the variation of the eccentricity modifies the ITT only if the transfer is through visual channels.
Clarke and Zaidel3 proposed a model in which all possible visual and motor connections are activated simultaneously during this task and result in multiple parallel processes terminating horse-race fashion by the one that completes processing first. Thus, the most efficient interhemispheric route determines ITT. Visual evoked potential estimates of ITT suggested that the motor channel is faster than the visual one, and that the latter is sensitive to visual parameters variation10, but methodological limitations (sampling rate of 3 msec per point, and use of the N160 component that may not be the most appropriate)11 may qualify these results. Another visual evoked potential study11 did not show any influence of the variation of eccentricity on the ITT, but in this case the variation was probably too small to be reliably detected, from 2.8[ring] to 1.8[ring]. Our data rather suggest that motor callosal channels are more efficient than visual ones when the stimulus presentation is more peripheral than 4 degrees. An indirect support to our result comes from a previous study measuring only post-operative ITT in a partial commissurotomy patient with an intact splenium of the corpus callosum and showing a large ITT at 10 degrees of eccentricity12. Visual callosal pathways have been thought to support the integrative function of perceptual continuity across the two visual hemifields16, which is reflected in a large callosal representation of the vertical meridian17. Recently, anatomical and physiological evidence has suggested that this may not be the only role of visual callosal pathways, which exhibit many characteristics of association connections18-19. The observed lengthening of the ITT at 8 degrees of eccentricity after partial commissurotomy may reflect transfer at different levels of the visual system and/or the involvement of callosal fibers of different size and conduction velocity20-21.
Unfortunately, this study cannot distinguish between motor and pre-motor channels. Early findings13 suggested a lack of direct callosal motor connections for the distal parts of the limbs (the most frequent motor response for this experiment is to press a button with one finger), but a later anatomical study has shown direct callosal connections for primary motor areas with distinct representations for separate fingers in monkeys.14 Given that premotor fibers occupy areas of the corpus callosum more rostral than motor fibers,8-9 future studies on patients with selective lesions to the genu may distinguish between motor or pre-motor channels.
Conclusion
The corpus callosum may be viewed as composed of multiple channels of communication between the two hemispheres. Each channel has its own morphological and functional properties. Converging data from neuroanatomical, neurophysiological, and neurobehavioral investigations like the present report can help to delineate the nature and interaction of these channels. Our study does not confirm the hypothesis that the callosal transmission time of visuo-motor information is always faster through the motor fibers rather than the visual fibers. Rather, it seems that the closer the visual stimulus to the vertical meridian, the smaller the difference between motor and visual transfer time.
Acknowledgements
We wish to thank the patient for his collaboration, Dr. Mirella Dapretto for comments and suggestions, Elicia David for research assistance, and an anonymous reviewer for comments and suggestions on a previous draft of the manuscript. This work was supported by an NIH grant NS 20187 to M.I. and E.Z., and by an NIMH RSA MH 00179 to E.Z.
References
1. Poffenberger AT. Arch. Psychol. 23, 1-73 (1912).
2. Marzi CA, Bisiacchi P, Nicoletti R. Neuropsychologia 29, 1163-1177 (1991).
3. Clarke JM, Zaidel E. Brain 112, 849-870 (1989).
4. Berlucchi G, Heron W, Hyman R, et al. Brain 94, 419-430 (1971).
5. Pandya DN, Rosene DL. Some observations on trajectories and topography of commissural fibers. In: Jeeves AG, eds. Epilepsy and the Corpus Callosum. New York: Plenum Press, 1985: 21-39.
6. Sprague JM, Berlucchi G, Rizzolatti G. The role of the superior colliculus and pretectum in vision and visually guided behavior. In Jung R, ed. Handbook of Sensory Physiology, Volume 7/3. Berlin: Springer, 1973: 27-101.
7. Vallar G, Sterzi R, Basso A. Neuropsychologia 26, 511-519 (1988).
8. Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore' F, Ptito M, Jasper HH, eds. Two hemispheres - one brain: Functions of the corpus callosum. New York: Liss, 1986: 47-73.
9. de Lacoste MC, Kirkpatrick JB, Ross ED. J. Neuropathol. Exp. Neurol. 44, 578-591 (1985).
10. Lines CR, Rugg MD, Milner AD. Exp. Brain. Res. 57, 89-98 (1984).
11. Saron CD, Davidson RJ. Behav. Neurosci. 103, 1115-1138 (1989).
12. Di Stefano MR, Sauerwein HC, Lassonde M. Neuropsychologia 30, 177-185 (1992).
13. Killackey HP, Gould HJ, Cusick CG, et al. J. Comp. Neurol. 219, 384-419 (1983).
14. Gould HJ, Cusick CG, Pons TP, Kaas JH. J. Comp. Neurol. 247, 297-325 (1986).
15. Rosenthal R, Rosnow RL. Contrast analysis : focused comparisons in the analysis of variance. New York, Cambridge University Press, 1985.
16. Antonini A, Berlucchi G, Lepore' F. J. Neurophysiol. 49, 473-483 (1983).
17. Kennedy H, Dehay C, Bullier J. J. Comp. Neurol. 247, 398-415 (1986).
18. Kennedy H, Dehay C. Behav. Brain Res. 29, 225-236 (1988).
19. Kennedy H, Meissirel C, Dehay C. Callosal pathways and their compliance to general rules governing the organization of corticocortical connectivity. In: Dreher B, Robinson S, eds. Vision and visual dysfunction, Vol. 3, Neuroanatomy of the visual pathways and their development. London: Macmillan, 1991: 324-359.
20. Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Brain Res. 598, 143-153 (1992).
21. Ringo JL, Doty RW, Demeter S, Simard PY. Cerebral Cortex 4, 331-343 (1994).