on the same topic, before and since.
Abstract. The source
of variability in the interval between action potentials has been identified
in a class of cat spinal motoneurons. The observed random fluctuations
in membrane potential (synaptic noise) together with an empirical description
of spike generation accurately predict the statistical structure of variability
observed to occur in the neuron's discharge.
The marked variability characteristic of most neurons' steady-state output
has been of considerable interest (1), because it has been believed to
add uncertainty to the information transmitted by the neuron and because
it may provide clues about the mechanisms underlying the transformation
of input to output. Despite considerable investigation, the sources of
variability in the interval between action potentials (the interspike interval)
have not yet been clearly identified for any type of neuron. One obvious
possible source is the haphazard fluctuations in membrane potential (2)
seen even in the quiescent neuron. The question arises as to whether such
input fluctuations (synaptic noise) can account for the output (interspike
interval ) variability. We have shown that synaptic noise, together with
a simple model for spike generation, can indeed account for variability
in the interspike interval in one class of cat spinal motoneurons.
Intracellular recordings (Fig. 1) from spinal motoneurons suggest
a mechanism by which synaptic noise could produce variability in the interspike
interval. During natural stimulation, or when a constant current is passed
through the recording electrode, many previously silent spinal motoneurons
discharged repetitively. Immediately after a spike the membrane repolarizes,
and then the membrane potential rises approximately linearly to the firing
level where another spike is generated (Fig. 1B). Superimposed upon this
ramp-like approach of the membrane potential to the firing level, however,
are random voltage fluctuations that look much like the synaptic noise
of the quiescent neuron. These fluctuations in voltage appear to cause
the membrane potential to reach the firing level at randomly varying times
instead of at a fixed time, as would be the case if no fluctuations were
present. According to this model then, synaptic noise produces variability
in the inter spike interval by causing variations in the time at which
the membrane potential first crosses the firing level.
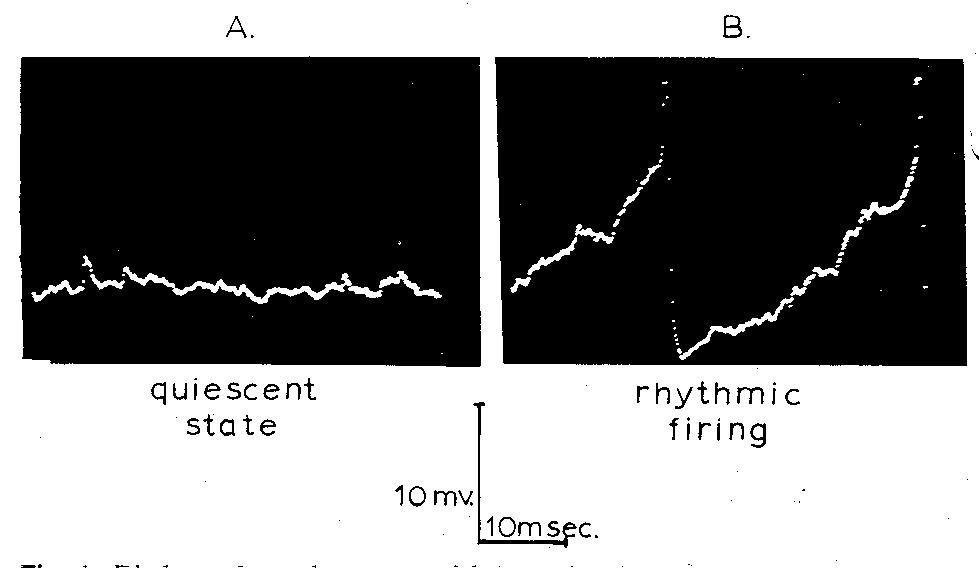
Fig. 1. Displays of membrane potential as a function of time showing
synaptic noise in a quiescent motoneuron (A) and the ramp-like approach
to the firing level with superimposed noise (B) when repetitive firing
was induced by current passed through the membrane. Note that the action
potentials are off scale in B. Because these records are displays of digitalized
data from LINC memory, the separate data samples are visible as dots where
the membrane potential was changing rapidly.
.
Although data from intracellular recordings are in qualitative
agreement with our proposed model, only quantitative evidence can establish
that more complicated assumptions are unnecessary. For example, it would
be very difficult, on the basis of mere inspection of records, to say that
some source of noise other than synaptic noise is not present, or that
synaptic noise does not interact with the ramp generating processes instead
of simply adding to the membrane potential as it approaches the firing
level.
Specifically, then, we find that the statistical structure of
variability in the interspike interval can be accurately predicted from
the observed properties of synaptic noise together with a simple model
of the spike-generating mechanism based on the assumption that the synaptic
noise simply adds to a linear rise of the membrane potential to a firing
level.
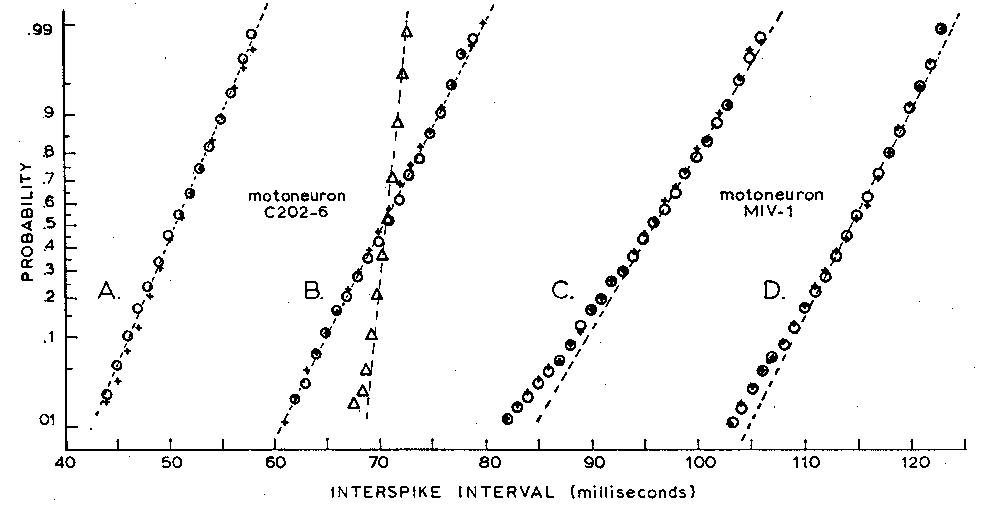
Fig. 2. Observed and predicted cumulative histograms of interspike
intervals from two motoneurons. The ordinate is a probability scale on
which a Gaussian distribution gives a straight line. Two sets of data from
a motoneuron whose firing level increased linearly with time after a spike
are shown (+) in (A) and (B), while results from a second motoneuron with
a constant firing level are shown (+) in (C) and (D). Predictions made
from synaptic noise and the observed characteristics of the spikegenerating
mechanism (open circles) are in good agreement with observed histograms;
if the time dependence of the firing level in (B) is ignored and if a constant
firing level is used for making the predictions, the predicted points (open
triangles) deviate markedly from the observed. For clarity of the illustration,
the points in (B) have been shifted 15 msec to the right along the time
axis.
Our data are based on observations of lumbosacral motoneurons in cats lightly
anesthetized with pentobarbital. Intracellular recordings were obtained
with low-resistance (2 to 8 megohm) glass microelectrodes filled with either
2.8M KCI or 2.5M potassium acetate. Signals were led through a negative
capacitance preamplifier, recorded on tape (frequency response flat from
0 to 5 khz), and finally played back into a digital computer (LINC) for
analysis. Analog voltages were converted into eight-bit words with a sampling
rate of 5 khz.
In the quiescent state all motoneurons exhibited the haphazard
fluctuations in membrane potential termed synaptic noise (Fig. 1A). When
current was passed through the recording electrode, the motoneuron fired
repetitively (Fig. 1B), showing a ramp-like approach to the firing level
and prominent, superimposed fluctuations in membrane potential which have
the same appearance as synaptic noise seen in the resting neuron.
To analyze the interspike interval variability, we first compiled
in the computer a list of intervals between successive action potentials
and screened these lists for segments of statistically stationary data
containing a minimum of 500 successive intervals. From about 100 neurons
that at the time of the experiment appeared to yield stable intracellular
recordings, we have selected four to report on here; seven segments of
data from these four motoneurons were chosen for detailed analysis.
Specifying completely the statistical structure of a neuron's
interspike-interval variability would require computation of the conditional
probability of a particular interval length given the entire past history
of that neuron's behavior. For our motoneurons, however, serial autocorrelograms
revealed that an interval is independent of all preceding intervals; in
this case, then, the interspike-interval histogram provides a complete
specification of a neuron's interval fluctuations.
Cumulated interspike interval histograms from four lists of intervals
yielded by two motoneurons are shown in Fig. 2. These plots are made on
a probability scale so that a Gaussian interspike-interval histogram would
give a straight line; histograms from cells not included in the illustration
were also approximately Gaussian [as opposed to exponential, multimodal,
and so forth (1)]
If the simple model of motoneuron behavior described earlier is
accurate, we should be able to predict these observed interspike-interval
histograms from measurements on the synaptic noise and on the average properties
of the spike-generating mechanism by simulating motoneuron behavior with
the digital computer. Simulation is preferable to the analytic approach
employed earlier (4) because idealizing assumptions inapplicable to some
neurons are thus avoided.
To make this simulation, a linearly rising "membrane potential"
was generated in the computer, and synaptic noise was added to this membrane
potential ramp. When the membrane potential plus noise first reached the
"firing level," the length of time since the previous crossing of the firing
level was stored, and the membrane potential was reset to begin the process
anew. In this way, a sequence of "interspike intervals" could be generated,
and their statistics could be compared with those of the observed neuronal
behavior.
Simulating a specific neuron's behavior requires that parameters
be selected for the deterministic spike-generation process and for the
synaptic noise. The values for the ramp slope, starting point, and firing
level were measured from records of membrane potential. "Synaptic noise"
for the simulation was derived from one of two sources. In one instance
the output of a commercial whitenoise generator was filtered to produce
noise with the same statistical structure (Gaussian probability density
and exponential autocorrelation function) as that observed in the neuron.
In the other cases, tape-recorded samples of synaptic noise from the quiescent
cell were used. Whatever the source of the noise voltage, it was sampled
at 5 khz and added to the "membrane potential" ramp generated within the
computer. It must be emphasized that all parameters of the simulation were
estimated from intracellular recordings from the particular neuron whose
behavior was being examined and that a neuron's own synaptic noise was
used in the simulation; no parameters were obtained from the interspike-interval
histograms we wished to predict.
Predicted interspike interval histograms for two motoneurons are
shown in their cumulated form in Fig. 2. The obviously good agreement between
predicted and observed histograms was confirmed by Kolmogorov-Smirnov goodness-of-fit
tests (5); for all motoneurons included in this report, more often than
one time in five, two observed histograms generated by the motoneuron itself
would differ as much as the observed and predicted histograms.
The sensitivity of this method to inaccurate assumptions is illustrated
by one of the cells (C202-6) included in Fig. 2. Although the firing level
was constant in some cells, it was observed to increase linearly with time
from the preceding spike in others. If this systematic rise in the level
was ignored--that is, if a constant firing level was adopted in the simulation--the
predicted interspike-interval histogram had a much smaller standard deviation
than that observed for the motoneuron (Fig. 2B). By arbitrarily increasing
the amplitude of the synaptic noise in the simulation by about 100 percent,
we could make the predicted and observed standard deviations of the interspike-interval
histograms match; however, their shapes were different, the predicted histogram
being negatively skewed. When the increase in firing level (approximately
50 microvolt/msec) observed in the motoneuron was included in the simulation,
the synaptic noise seen in the cells gave an accurate prediction of the
observed interspike-interval histograms (circles, Fig. 2, A and B).
Although synaptic noise appears wellestablished as the dominant
source of interspike-interval variability in at least some cells, the question
of whether additional noise sources make important contributions in other
cells naturally arises. This is a difficult question to answer, however,
since any failure to predict accurately the observed interspikeinterval
histogram does not imply that sources of variability other than synaptic
noise were the cause of failure. For example, if the firing level depends
in some complicated way on the entire history of the membrane potential
path, that is, if voltage fluctuations that do not cross the firing level
still have some effect on the state of the neuron, then our predictions
will fail because the model of the (deterministic) spike-generating mechanism
is inadequate and not because something other than synaptic noise is a
major source of noise. To identify a second major noise source, then, it
must be shown ,that including its observed properties in the stimulation
leads to accurate prediction of the interspike interval histogram. In one
cell, we found the slope of the membrane potential ramp to vary randomly
from interval to interval. Since including this additional observed behavior
in the simulation leads to accurate prediction of the interspike-interval
histogram, we have discovered at least one instance where a source other
than synaptic noise (presumably variability arising in the spike-generating
mechanism) is important. It is interesting to note that we have not yet
found neurons in which threshold variability has appeared to contribute
significantly to the interspike-interval variability.
WILLIAM H. CALVIN
CHARLES F. STEVENS
Department of Physiology and Biophysics,
University of Washington School of Medicine, Seattle 98105
References and Notes
1. G. P. Moore, D. H. Perkel, J. P. Segundo, Annu. Rev. Phvsiol.
28, 493 (1966).
2. L G. Brock, J. S. Coombs, J. C. Eccles, J. Physiol. 117, 431
(1952); B. Katz and R. Miledi, ibid. 168, 389 (1963); R. Granit, J. O.
Kellerth, T. D. Williams, ibid. 174, 435 (1964); T. G. Smith, Jr., Proc.
Annu. Conf. Eng. Med. Biol. 18th 7, 120 (1965); R. E. Burke and P. G. Nelson,
Science 151, 1088 (1966); S. Watanabe and O. D. Creutzfeldt, Exp. Brain
Res. 1, 48 (1966); S. Hill, C. E. Polkey, T. D. Williams, in Muscular
Aflerents and Motor Control, R. Granit, Ed. (Wiley, New York 1966),
p. 3630.
3. We thank Drs. J. W. Woodbury and A. M Gordon for the use of
their LINC.
4. W. H. Calvin and C. F. Stevens, Proc. Annu. Cont. Eng. Med.
Biol. 18th 7, 118 (1965).
5. D. A. Darling, Ann. Math. Stat. 28, 823 (1957).
6. This work was supported by NIH grant NB 05934. Computer facilities
used were supported by NIH grant FR 00150.
3 January 1967
WCalvin@U.Washington.edu
|| Home Page || Calvin
publication list || My Science Surf
column || The
Calvin Bookshelf || scan and web, 7 Jan 97


