Acknowledgement: This research was funded in part by a grant to A.M. from the Harry Frank Guggenheim Foundation.
Address comments to:-
Phone: (315) 443-9310
Fax: (315) 443-1075
email: amazur@syr.edu
Keywords: testosterone, cortisol, dominance, competition, sex
Testosterone (T) and cortisol (C) were assayed from saliva samples given by young men (n = 28) and women (n = 32) before, during, and after competing with a same-sex partner in a video game. The T response to the competition is different in each sex; the C response is the same. Male results confirm prior reports of a pre-contest rise in testosterone. Male results did not confirm previous findings that after a contest, the testosterone of winners is higher than that of losers, perhaps because the video game contest produced little mood difference between male winners and losers. Unlike male testosterone, female testosterone generally decreased throughout the experiment. Trends in T and C are parallel in women but not in men. Apparently T works differently in competition between men than between women.
Males holding high rank in primate dominance hierarchies have a reproductive advantage over low ranking males (Ellis 1995). Since testosterone (T) is implicated in dominance competition among males (Mazur 1985; Kemper 1990; Mazur and Booth in press), it presumably facilitates their acquisition of females and other resources, and it may fuel aggressiveness in males (Daly and Wilson 1988).
Competitiveness is useful for females too, especially in vying for the support of males and in protecting and providing for offspring (Hrdy 1981). Therefore T may play a role in female dominance, perhaps operating similarly to the hormonal mechanism in males. Presently we understand less about T in females than in males (Mazur and Booth in press).
In males, the relationship between T and dominance is known to be reciprocal. Not only does T affect dominance, but dominance competition affects T level. Studies of male athletes show T varying in predictable ways both before and after competitive matches. First, athletes’ T rises shortly before their matches, as if in anticipation of the competition (Campbell, et al. 1988; Booth, et al. 1989). Second, for one or two hours after the match, T levels of winners are high relative to those of losers (Mazur and Lamb 1980; Elias 1981; Campbell, et al. 1988; Booth, et al. 1989).
The above results were obtained in physically taxing sports (tennis and wrestling), which are convenient arenas in which to test hypotheses about the involvement of T in face-to-face competition. However, theorists who relate T to everyday social interaction are interested primarily in less vigorous competition and symbolic changes in social status (Kemper 1990; Mazur and Booth in press). Additional studies show the same pattern of male T responses during nonphysical contests or ritual status manipulations. First, T rises shortly before chess matches (Mazur, et al. 1992) or laboratory contests of reaction time (Figure 1 of Gladue, et al. 1989), and in subjects confronted with a symbolic challenge from an insult (Nisbett and Cohen 1996). Second, T levels of winners are high relative to those of losers following chess matches (Mazur, et al. 1992) and contests of reaction time (Gladue, et al. 1989). Mazur and Lamb (1980) found that T of medical students rises after their graduation ceremony when their mood is elated. Thus, the T pattern appears in nonphysical as well as physical competition, and in response to symbolic challenges and status changes among men.
The rise in T following a win is associated with the subject’s elated mood of victory or elation (Booth, et al. 1989). If the mood elevation is absent, or subjects do not regard the win as important, then the rise in T does not occur (Mazur and Lamb 1980). In both winners and losers of a lottery, T elevation is correlated to positive mood change (McCaul, et al. 1992). When Salvadore, et al. (1987) did not obtain the win-loss effect on T among wrestlers, they noted that their subjects did not take the matches seriously.
Studies to date have used male subjects, who produce more T than females. There is considerable theoretical speculation about whether or not T responds the same way to status competition in both sexes (Mazur 1985; Kemper 1990). Here we compare the T responses of men and women during nonstrenuous same-sex competition in a video game. Do men and women have similar T responses to competition?
Male T is produced primarily in the testis and secondarily in the adrenal
cortex. In women, T is produced about equally in the ovaries and the adrenal
cortex (Miller and Tyrrell 1995). The adrenal cortex is also the production
site for cortisol (C), which is increased during episodes of physical or
psychological stress (Sapolsky 1992; Kirschbaum and Hellhammer 1994a).
It is plausible that some mode of activating the adrenal cortex produces
T and C simultaneously. If so, changes in T would parallel changes in C
in women, because their adrenal cortex is the primary source for
both hormones. Since male T comes mostly from the testis, male T would
not be expected to covary with C production. Do T and C change in parallel
for women but not for men?
Subjects and Procedure
Twenty-eight males and 32 females, ranging in age from 17 to 35 years (mean = 20, median = 19), mostly undergraduates and of diverse race and nationality, were recruited through an advertisement in the student newspaper asking for subjects for a "social psychology study using video games," and by word of mouth. Subjects participated as same-sex pairs. An ideal design would have included mixed-sex pairs too, however this was precluded by the considerable added expense. Also, mixed-sex pairs of college students raise romantic connotations, which may confound the comparison of sex-specific responses.
Two subjects were seated on the same side of a long table so they could see a video display on the other side of the table. An opaque screen separated the subjects to allow privacy. The experimenter explained, "I want to trace your body chemistry while you are playing a video game. I check your body chemistry from saliva samples, so I’ll ask you to give me five saliva samples over the next hour." Subjects then signed written consent forms and were paid $5 for their participation.
Only after providing the first saliva sample were subjects told that they would compete with each other in the games. The experimenter stressed that they are opponents and should seriously try to win all their games. Following a demonstration of the game, which resembles Ping-Pong (trademark "TV Scoreboard," Radio Shack), and a short practice session, subjects gave a second saliva sample. Then they were told, "You’ll play a five game tournament; whoever takes three games is the winner. Now really try to win. OK, I’ll start the first game."
After completing the first game, subjects gave a third saliva sample. Subjects then played until a winner was determined, at which point the experiment shook hands with the winner and announced, "I declare (name) the winner. Congratulations!" Subjects then waited for two minutes to allow time for a hormonal response before giving the fourth saliva sample. Subjects were then asked to describe their feelings and to evaluate their own and their opponent’s performances as video Ping-Pong players. Ten minutes after sample #4, and following a full debriefing, subjects gave the fifth saliva sample, completing the run.
Table 1 summarizes this schedule and shows the mean time for each event
and saliva sample in "running" minutes, beginning with time zero for sample
#1.
________________________________________________________________________
Table 1. Schedule of Events and Saliva Samples
Time
(minutes) Event
0 Begin collection of saliva sample #1.
4 E explains that Ss will be competitors, E demonstrates game, Ss practice.
8 Begin collection of saliva sample #2.
11 Ss play first game.
15 Begin collection of saliva sample #3.
18 Ss play remaining games.
28 E declares winner, Ss wait for two minutes.
30 Begin collection of saliva sample #4.
33 Ss report feelings and evaluate play.
43 Begin collection of saliva sample #5.
46 Conclusion
____________________________________________________________________________
Evaluation and Mood Measurements
In the ten minutes between finishing saliva sample #4 and beginning to collect #5, the experimenter asked subjects to "please write a couple of sentences describing your feelings right now, and rate your performance as a video Ping-Pong player. Also, how well do you think your opponent played?" These prose responses were later transcribed with the sex of the subject disguised. Two blind coders independently judged the responses as reflecting success or failure in the tournament, rating each on a 1-7 scale where "7" indicates maximum success. The Pearson correlation between ratings is r = .76 (p = .0001). The average of each subject’s two ratings defines the variable SUCCESS.
Subjects were also asked to "write how you feel at this moment, on a scale from one to ten, where ‘one’ means you feel poorly and ‘ten’ means you feel great." This numerical self rating is designated MOOD.
Hormone Measurement
Concentrations of T and C in saliva are highly correlated with concentrations of the respective free hormones in blood (Wang, et al. 1981; Dabbs 1990, 1991; Kirschbaum and Hellhammer 1994a, 1994b).
At each sampling, subjects gave about five milliliters of saliva by spitting into a plastic storage vial. Nearly all subjects chewed sugarless gum to stimulate saliva. The average time for collecting each sample was about three minutes. Samples were frozen.
Samples were radioimmunoassayed at the Behavioral Endocrinology Laboratory (BEL) at The Pennsylvania State University. The T assay used a BEL modification of a kit produced by ICN (Costa Mesa, CA) and had a lower limit of sensitivity of .024 ng/ml. The C assay, using a modification developed by Megan Gunnar, University of Minnesota Hospital, of a kit produced by Pantex (Santa Monica, CA), had a lower limit of sensitivity of .05 ug/dl. All samples from a given individual were assayed in duplicate in the same batch (except for two subjects whose C samples were inadvertently split between two batches). Within-assay coefficients of variation, calculated from the duplicates, are 3% for male T, 7% for female T, 4% for male C, and 5% for female C. Between-assay coefficients of variation, based on internal controls, are 6% for male C and 9% for female C.
In order to control for any sex difference in the magnitude of each hormone, and to minimize between-subject variation, each subject’s raw hormone values were divided by his or her highest measured level (Mazur and Lamb 1980; Mazur, et al. 1992). These normalized values, which range from above zero to 1.0, are used in the analysis unless otherwise stated. An additional advantage of this procedure is that, by thus anchoring mean maxima near 1.0, we aid visual comparison of time trends between sexes and hormones. Analysis using raw hormone values shows similar results.
Calculation of repeated-measures ANOVA requires that all hormone values be present (Freund, Littell, and Spector 1986). Saliva sample #2 for one male winner was discarded immediately after collection because of severe blood contamination. Missing T and C values for this sample were replaced by the mean normalized T and C values of sample #2 for the 13 other male winners. Also missing, because of error or insufficient saliva, are one other male C value, and both hormone values for four female samples; these were replaced by mean normalized values for the other subjects, matched by sample number, sex, and winner/loser status.
T and C show diurnal variation in both sexes, being higher in the morning than the afternoon or evening (Dabbs 1990; Kirschbaum and Hellhammer 1994b). In order to reduce this source of variation, all subjects were run between 1:00 and 10:00 P.M. This control was successful for T levels, which did not vary with time of day. Men’s C levels, but not women’s, were significantly higher earlier in the afternoon.
Female subjects were asked the number of days since the beginning of their last menstrual period. Information was not obtained on use of contraceptive pills, which have no discernible effect on salivary C (Kirschbaum and Hellhammer 1994b) but may affect T.
Consistent with the finding of Dabbs (1990), we found neither T nor C to vary with the day of the menstrual cycle. Normally T would be expected to vary with cycle, but the relationship may have been masked by subjects’ unreliable reports of their cycle, use of contraception, or a high incidence of irregular periods among college students.
Hemastix Measurement.
T and C are more concentrated in blood than in saliva, so blood contamination may conceivably exaggerate their readings from saliva. Following Gladue, et al. (1989), we tested for the presence of blood with Ames Hemastix strips (Miles Inc., Elkhart IN). All saliva samples gave positive Hemastix results, suggesting the presence of blood, usually at low levels. Subjects who reported bleeding gums had significantly higher Hemastix scores than nonbleeders on four of five saliva samples. Hemastix scores were fairly consistent across saliva samples for a single subject, the between-sample correlation averaging about r = .60 (p = .0001). Scores decreased with succeeding samples, apparently because continued expectoration clears the mouth of most trace blood.
Does the presence of trace blood affect T or C level? For men, the correlation between Hemastix score and either raw T or raw C level was insignificant in all samples. For women, the correlation between Hemastix score and raw T was both sizable and significant (r = .50, p = .003) in sample #4, but smaller and insignificant in the other samples; Hemastix was not significantly correlated to raw C. Results reported below are virtually unchanged when Hemastix score is included as a covariate.
Mean T for men, across the five saliva samples respectively, is 9.5, 10.0, 9.8, 9.9 and 10.0 ng/dl (SD = 2.6-2.8). As expected, these are significantly higher, sample by sample (p = .0001), than mean female T across the five samples: 2.6, 2.4, 2.2, 2.1 and 2.1 ng/dl (SD = 0.9-1.1). These are normal salivary values for each sex (Dabbs 1990). T levels tend to be fairly consistent across samples for both sexes; between-sample correlations among men range from r = .83 to .96, and among women from r = .70 to .86 (p = .0001).
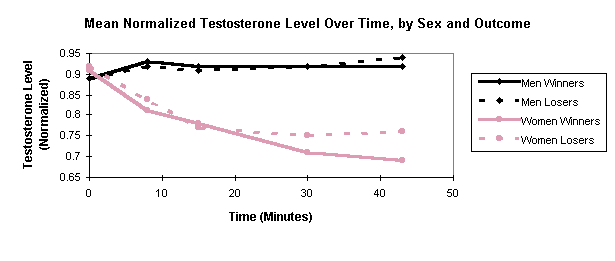
In Figure 1, mean normalized T is plotted as a function of time, using
separate graphs for male winners (n = 14), male losers (n = 14), female
winners (n = 16), and female losers (n = 16). It is apparent from inspection
that T trends differ considerably across the sexes. Male T (for both winners
and losers) stays high while female T generally drops with time. An ANOVA
treating the five successive T values as repeated measures shows that the
interaction of timeXsex is highly significant (p = .0005), as is
the main effect of sex (p = .0001).

Male T (for both winners and losers) shows the expected prematch rise from saliva #1 (at 0 minutes, before subjects knew they would compete) to saliva #2 (at 8 minutes, after subjects learned of the competition). For men, a paired-comparison t-test of the change in normalized T, from saliva #1 to #2, is significant (p = .04 using a one-tailed test, which is justified because it replicates an established result). None of the other adjacent changes in male T (e.g. from saliva #2 to saliva #3) approaches significance. However, it is noteworthy that 25% of the men had higher T values in saliva #1 than in saliva #2, so the anticipatory rise, while present in the aggregate, is not highly reliable across subjects. In contrast, women showed no anticipatory prematch rise in T, as is apparent in Figure 1. In fact, a paired-comparison t-test showed a significant reduction in normalized T (p = .02) from saliva #1 to #2; other adjacent changes are insignificant.
Contrary to expectation, the graph shows no tendency (in either sex) for winners to have higher T than losers after the match (samples 4 and 5). Repeated-measures ANOVAs, applied to each sex separately, show the interaction of timeXwin/loss to be insignificant. Simple t-tests, applied to each saliva sample for each sex, also show no significant differences between winners and losers.
Summing up, there was a clear sex difference in T response to competition. In women, T generally declined, showing neither an anticipatory rise before the match, nor a difference between winners and losers after the match. In men, T did show the expected prematch rise, however, contrary to expectation, there was no significant difference in T between winners and losers after the match.
Cortisol Results
In each saliva sampling, women have significantly higher C than men (p < .01). Mean female C, across the five samples respectively, is 326, 303, 268, 228 and 201 ng/dl (SD = 117-234). Mean male C across the five samples is 197, 187, 151, 123 and 116 ng/dl (SD = 80-146). Standard values for each sex are not well determined, although it is unusual for females to exceed males (Kirschbaum and Hellhammer 1994b).
For women, C levels are consistent across samples, their between-sample correlations ranging from r = .73-.92 (p = .0001). Male C levels are more variable, with between-sample correlations ranging from r = .19 (ns) to .88 (p = .0001).
Since women produce as much T from the adrenal cortex as the ovaries, and their C is produced by the adrenal cortex (Miller and Tyrrell 1995), female values for the two hormones might be expected to be correlated. Each hormone is measured five times, allowing 25 possible correlations between raw T and raw C. For women, 16 or these correlations are significant at p < .01 (r > .43), and five more reach p = .05. The picture is very different for men: none of their 25 correlations between C and T is significant, and none exceeds r = .19.
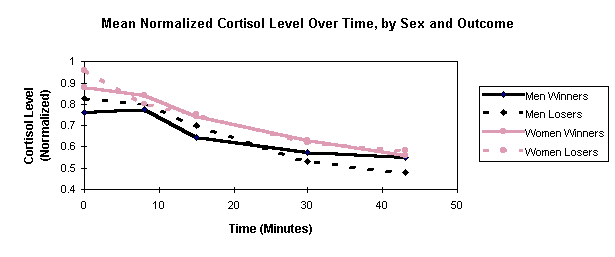
Figure 2. Mean Normalized Cortisol Level Over Time, by Sex and Outcome
In Figure 2, mean normalized C is plotted as a function of time, using separate graphs for each combination of sex and outcome. C trends are similar for winners and losers of both sexes: C declines with time. An ANOVA treating the five successive C values as repeated measures shows neither sex nor the interaction of timeXsex approaching significance.
Figure 2 shows no difference in C between winners and losers (for either sex). Repeated-measures ANOVAs, applied to each sex separately, show the interaction of timeXwin to be insignificant. Simple t-tests, applied to each saliva sample for each sex, also show no significant differences between winners and losers.
Success and Mood Results
Subjects’ evaluation of the play (SUCCESS, as rated by coders) and their self rating of their feelings after the tournament (MOOD) are correlated for women (r = .57, p = .001) but not for men (r = .12, p = .53).
Table 2 shows mean scores on SUCCESS and MOOD for winners and losers,
by sex. Among both men and women, winners’ descriptions were rated significantly
higher on SUCCESS than were losers’ descriptions (p = .0001, t-test). But
the sexes show different patterns on MOOD. Among women, winners report
better MOOD than do losers, an almost significant difference of 1.1 (p
= .06), whereas among men the difference between winners and losers is
only 0.4. Thus, the experience of winning versus losing essentially failed
to affect self-rated moods of the men.
Table 2. Mean SUCCESS and MOOD for Winners and Losers, by Sex
|
|
|
|||
|
|
|
|
|
|
|
Winners: |
|
|
|
|
|
|
|
|
|
|
| Losers: |
|
|
|
|
|
|
|
|
|
|
| Probability that difference of means = zero: |
|
|
|
|
Neither SUCCESS nor MOOD show any significant pattern of correlations
with T or C, for either sex.
T response to a video game competition is different in male dyads than in female dyads.
As expected, male T rose before the video contest, as if in anticipation of the competition. However our male subjects did not show the expected postmatch response: male winners’ T was no higher than that of losers. This null result may be due to the failure of the video tournament to elicit different moods in male winners and losers, an interpretation consistent with previous work associating T changes with mood changes.
Our advertisement for subjects mentioned that video games would be used in the experiment. Judging from casual comments by subjects, we apparently recruited more experienced gamers among male than female applicants. Our Pong game is archaic by today’s standards, its graphics simply a moving dot (the ball) crossing back and forth over a line (the net). Perhaps our experienced male gamers thought Pong too "low tech" to be engaging, whereas the women, who were less habitual players, found it more interesting.
Female winners did report more positive mood than losers, yet even with this precondition met, their postmatch T did not surpass that of losers. Nor did women show any anticipatory prematch rise in T. Thus, female T did not respond to either the announcement of competition or the experience of winning/losing.
Taken at face value, these results suggest that T has a different relationship to competition in men than in women. An alternate explanation, that female winners and losers did not perceive themselves in competition, seems implausible in view of their differentiation on SUCCESS and MOOD (Table 2). Also, female C was consistently higher than male C, and since no general sex difference in C is recognized (Kirschbaum and Hellhammer 1994b), this suggests that women were experiencing a higher level of stress during the experiment, seemingly inconsistent with their taking the game less seriously than men.
T and C trends show parallel declines in women but not in men. This parallelism is consistent with a sex difference in physiology. In women, T is largely produced in the adrenal cortex, the same organ that produces C. Perhaps the adrenal cortex was stimulated by the stress of entering the laboratory to produce both hormones, and then they declined together with habituation. Arguing against this is the finding that ACTH, injected intravenously, produces in women elevated C but no elevation in T (Lashansky, et al. 1991). Possibly T and C are jointly increased in women by some other mechanism. In any case, T and C are less likely to be entrained in men, who primarily produce the two hormones in different organs.
Heightened T is associated with dominating and assertive behavior in both sexes (Mazur and Booth in press; Cashdan 1995). In males, the rise of T that precedes competition should facilitate their dominating actions once the contest begins. Of course, women contestants will also attempt to dominate, but apparently they must do it without any hormonal consequences. On the other hand, if women under stress experience rising T (in parallel to rising C), and thereby obtain a hormonal boost to their assertiveness, then here is a mechanism for dominance under duress that does not operate in men.
Booth, A., Shelley, G., Mazur, A., Tharp, G., and Kittok, R. Testosterone, and winning and losing in human competition. Hormones and Behavior 23: 556-571. 1989.
Campbell, B., O’Rourke, M., and Rabow, M. Pulsatile response of salivary testosterone and cortisol to aggressive competition in young males. Paper presented at Annual Meeting of the American Assn. of Physical Anthropologists, Kansas City. 1988.
Cashdan, E. Hormones, sex, and status in women. Hormones and Behavior 29: 354-366. 1995.
Dabbs, Jr., J. Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiology and Behavior 48: 83-86. 1990.
Dabbs, Jr., J. Saliva testosterone measurements: Collecting, storing, and mailing saliva samples. Physiology and Behavior 49. 1991.
Daly, M. and Wilson, M. Evolutionary social psychology and family homocide. Science 242: 519-524. 1988.
Elias, M. Serum cortisol, testosterone, and testosterone-binding globulin responses to competitive fighting in human males. Aggressive Behavior 7: 215-224. 1981.
Ellis, L. Dominance and reproductive success among nonhuman animals. Ethology and Sociobiology 16: 257-333. 1995.
Freund, R., Littell, R., and Spector, P. 1986. SAS System for Linear Models. Cary, NC: SAS Institute. 1986.
Gladue, B., Boechler, M., and McCaul, K. Hormonal response to competition in human males. Aggressive Behavior 15: 409-422. 1989.
Hrdy, S. The Woman That Never Evolved. Cambridge, MA: Harvard University Press. 1981.
Kemper, T. Social Structure and Testosterone. New Brunswick, NJ: Rutgers University Press. 1990.
Kirschbaum, C. and Hellhammer, D. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 19: 313-333. 1994a.
Kirschbaum, C. and Hellhammer, D. Methodological aspects of salivary cortisol measurement. Pp. 19-25 in C. Kirschbaum, G. Read, and D. Hellhammer (Eds.), Assessment of Hormones and Drugs in Saliva in Biobehavioral Research. Seattle: Hogrefe & Huber. 1994b.
Lashansky, G., Saenger, P., Fishman, K., Gautier, T., Mayes, D., Berg, G., Di Martino-Nardi, J., and Reiter, E. Normative data for adrenal steroidogenesis in a healthy pediatric population. Journal of Clinical Endocrinology and Metabolism 73: 674-686. 1991.
Mazur, A. A biosocial model of status in face-to-face primate groups. Social Forces 64: 377- 402. 1985.
Mazur, A. and Booth, A. Testosterone and dominance in men. Behavioral and Brain Sciences. In press.
Mazur, A., Booth, A., and Dabbs, Jr., J. Testosterone and chess competition. Social Psychology Quarterly 55: 70-77. 1992.
Mazur, A., and T. Lamb. Testosterone, status, and mood in human males. Hormones and Behavior 14: 236-246. 1990.
McCaul, K., Gladue, B., and Joppa, M. Winning, losing, mood, and testosterone. Hormones and Behavior 26: 486-504. 1992.
Miller, W. and Tyrrell, J. The Adrenal Cortex. Pp. 555-712 in P. Felig, J. Baxter and L. Frohman (Eds.), Endocrinology and Metabolism, 3rd Edition. New York: McGraw Hill. 1995.
Nisbett, R. and Cohen, D. Culture of Honor. Coulder, CO: Westview Press. 1996.
Salvador, A., Simon, V., Suay, F., and Llorens, L. Testosterone and cortisol responses to competitive fighting in human males. Aggressive Behavior 13: 9-13. 1987.
Sapolsky, R. Neuroendocrinology of the stress-response. In J. Becker, S. Breedlove, D. Crew (Eds.), Behavioral Endocrinology (pp. 287-324). Cambridge, MA: MIT Press. 1992.
Wang, C., Plymate, S., Nieschlag, E., and Paulsen, C. Salivary testosterone in men: Further evidence of a direct correlation with free serum testosterone. Journal of Clinical Endocrinology and Metabolism 53: 1021-1024. 1981.