Ingestion of Amniotic Fluid by Postpartum Rats
Enhances Morphine Antinociception Without
Liability to Maternal Behavior
J. A. TARAPACKI, M. PIECH AND M. B. KRISTAL1
Behavioral Neuroscience Program, Department of Psychology, State University of New York at Buffalo,
Buffalo, NY 14260-4110 e-mail: KRISTAL @ acsu.buffalo.edu
Received 1 April 1993
| Parturition
Maternal behavior |
Opioids
Analgesia |
Morphine
Placentophagia |
Pain
POEF |
Tail-flick test | Amniotic fluid |
AMONG most nonaquatic placental mammals, placentophagia, the ingestion of birth membranes and fluids, is typical during delivery (16,17). One of the effects of this behavior is the enhancement of opioid-mediated analgesia (16). By itself, the ingestion of placenta or amniotic fluid does not produce analgesia (20,23), nor does it appear to enhance nonopioid-mediated analgesia (19). It does, however, enhance analgesia produced by injection of morphine (1,6,18-20,22,23,31), foot shock (22), vaginal/cervical stimulation (1,7,23), and late pregnancy (21). The relevance of this phenomenon to normal parturition is supported by findings that opioid levels (8,9,12,14,26,27,34) and pain thresholds (2,10,11) are elevated during pregnancy and show peaks at or near delivery.
One question that this strategy of potentiating the analgesic effect
of the opioids available at parturition raises is whether it offers some
advantage over increased opioid production or release. The possibility
has been suggested (16) that it may be a way of providing the benefit of
greater analgesia with less risk of the disruption of maternal behavior
that a number of studies (4,13,15,24,25,29) have shown to be a consequence
of excessive levels of opioids. Those studies used retrieval and grouping
of pups as a combined index of the quality of maternal behavior. The choice
of these criteria is supported by the observation that the test rats exhibiting
these components of maternal behavior, especially retrieval, also exhibit
most if not all the other components of maternal behavior (e.g., crouching
over pups in a nursing posture; licking and cleaning of the pups) (28).
In addition, the active spatio-motoric nature of retrieval and pup grouping
makes these components of maternal behavior susceptible to interference
by opioid action. Consequently, we adopted the same set of criteria (retrieval
and pup grouping) for testing our hypothesis in the present experiment.
METHOD
Subjects
A total of 46 nulliparous female Long-Evans rats averaging 296 ±
2.4 g at the time of testing were used as subjects. The rats were maintained
on a 14 h on/10 h off light cycle with the light phase beginning at 0600
(EST). Solid food (Agway Prolab Rat/Mouse/Hamster Formula 3000) and tap
water were available ad lib except during testing. The females were mated
by placing them with experienced males. Pregnant females were moved into
clear plastic cages (46 X 25 X 21 cm) fitted with wire tops and containing
approximately one liter of wood shavings. The date of delivery was designated
as Day 1 of lactation if birth of the first pup occurred prior to 1400
h; otherwise, the designation was assigned to the following day. Testing
was conducted between 1400 and 1800 on Day 3 of lactation.
Apparatus
During testing, the experimental and control fluids, amniotic fluid and physiological saline, were delivered orogastrically through an 11.5-cm length of PE-160 tubing fitted to a blunted 16-ga hypodermic needle on a glass syringe. Amniotic fluid was obtained surgically from Day 21 pregnant donors euthanized with CO2. Both the amniotic fluid and the saline were frozen at -20° C until needed. The fluids were warmed to 37° C for intubation.
Pain thresholds were measured while the rats were restrained in opaque, polyvinyl tubes (5 X 2 cm). Testing was conducted with a tail-flick latency algesiometer that has been described previously (22) and is similar to the apparatus that is standard in other laboratories (3,5,30). The dependent variable was the number of seconds it took for a rat to move its tail from the heated stimulus field (tail-flick latency, or TFL). The mean of the last three of four consecutive trials conducted at 30-s intervals served as the test score. To prevent tissue damage, trials were terminated at 8 s if no response occurred.
All subjects were habituated to both the intubation and the restraint
procedures prior to testing.
Procedure
Nine rats were assigned to each of four groups. To minimize any possible effect of litter size on maternal performance, the assignments were made so that the distribution of litter sizes (10.8 ± 0.4 pups per litter) were as similar as possible among the groups. The four groups formed a 2 X 2 experimental design: Drug [morphine, vehicle] X Enhancer [amniotic fluid, saline]. The rats in two of the groups received 2 mg/kg morphine sulfate (IP) followed by an orogastric infusion of 0.25 ml of either amniotic fluid or saline. The 2 mg/kg morphine dose was selected after we obtained pilot results that suggested it would be just below threshold for antinociception in the conditions of our test. The rats in the other two groups were injected with saline (1 ml/kg, IP) before receiving an orogastric infusion of either amniotic fluid or saline.
In order to provide another comparison for the level of analgesia expected from the subjects in the group that would be receiving both morphine and amniotic fluid, a separate group of ten rats (10.9 ± 1.0 pups per litter) was treated with 3 mg/kg (IP) morphine in conjunction with orogastric saline infusion.
For 3 h prior to being tested, and during the period of the tests, the rats were deprived of food and water to avoid any confound that might be introduced by absorption of infused fluids into the contents of a full stomach. Thirty min before testing began, the rats were weighed and the pups were removed from the cages.
In all cases, the experimenter conducting the tests was blind to the condition of the rats. Testing began with an assessment of the subject's baseline TFL followed by the morphine or vehicle injection. Fifteen min after the injection, the rat was infused with the enhancer or its control. Ten min later, the subject's TFL was measured once more. For purposes of analysis, this latency was converted into percent change from baseline.
The maternal-behavior test took place immediately after the second TFL
test. The subject was placed in her nest and six of the pups from her litter
were returned to the cage at the end opposite the nest site. The subject's
behavior was monitored until either all six pups were retrieved and grouped
together or until 15 min had elapsed. The subject was scored as either
having completed pup retrieval if all six pups were moved to the nest site,
or as having failed to complete pup retrieval within the test period.
RESULTS
Pain Threshold
The effect of ingestion of amniotic fluid on pain threshold was assessed with a 2-way ANOVA followed by a Newman - Keuls pairwise comparison. Comparisons with the 3 mg/kg morphine group were examined with t-tests. The baseline mean TFLs for the various groups varied from 3.75 ± 0.07 s to 3.94 ± 0.06 s; there were no significant differences among the groups, p > 0.05.
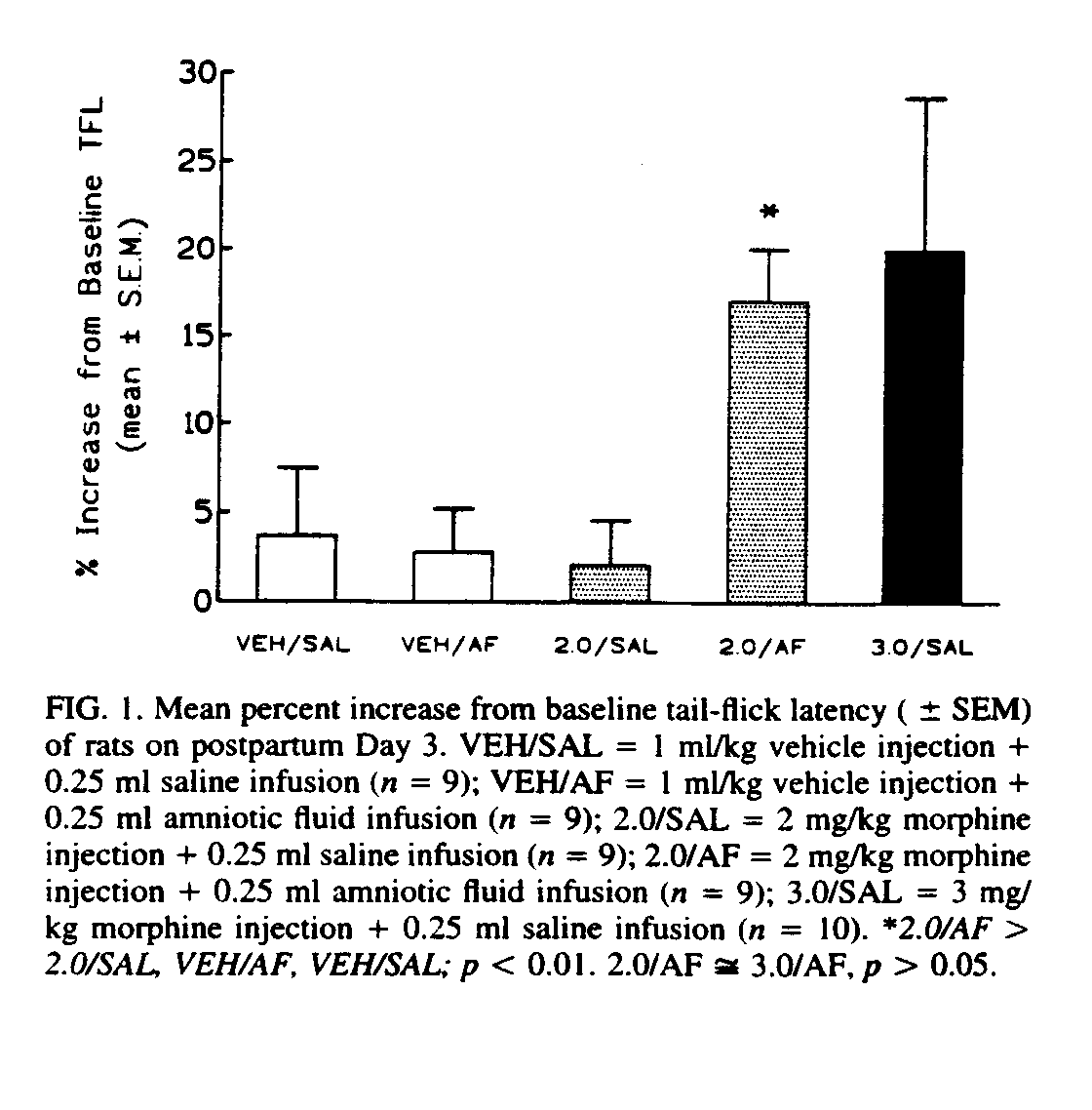
The results from the groups injected with 2 mg/kg morphine or with saline are presented in Fig. 1.
The ANOVA showed a significant Drug X Enhancer interaction, F(l, 32) = 7.04, p < 0.05, as well as significant main effects of both Drug, F(1, 32) = 4.46, p < 0.05, and Enhancer, F(1, 32) = 5.52, p < 0.05. The Newman - Keuls test revealed that these effects were entirely due to the group that had received both morphine and amniotic fluid (mean percent change from baseline = 17 02% ± 3.03%). This group differed significantly (p < 0.01) from each of the other groups (morphine + saline = 2.05% ± 2.50%; vehicle + amniotic fluid = 2.76% ± 2.43%; vehicle + saline = 3.67% ± 3.81%).
The group receiving a 2-mg/kg morphine injection in conjunction with an infusion of amniotic fluid (17.02% ± 3.03%) did not differ significantly from the group receiving a 3-mg/kg morphine injection in conjunction with a saline infusion (19.96% ± 8.69%, p > 0.05). The rats given 3 mg/kg morphine did differ significantly, however, from the rats given 2 mg/kg morphine plus saline, t(17) = 1.82, p < 0.05, and from both groups of vehicle-injected rats, t(26) = 2.28, p < 0.05). Ingestion of amniotic fluid, therefore, elevated pain thresholds (TFLs) in rats receiving 2 mg/kg morphine to a level similar to that produced by 3 mg/kg morphine alone.


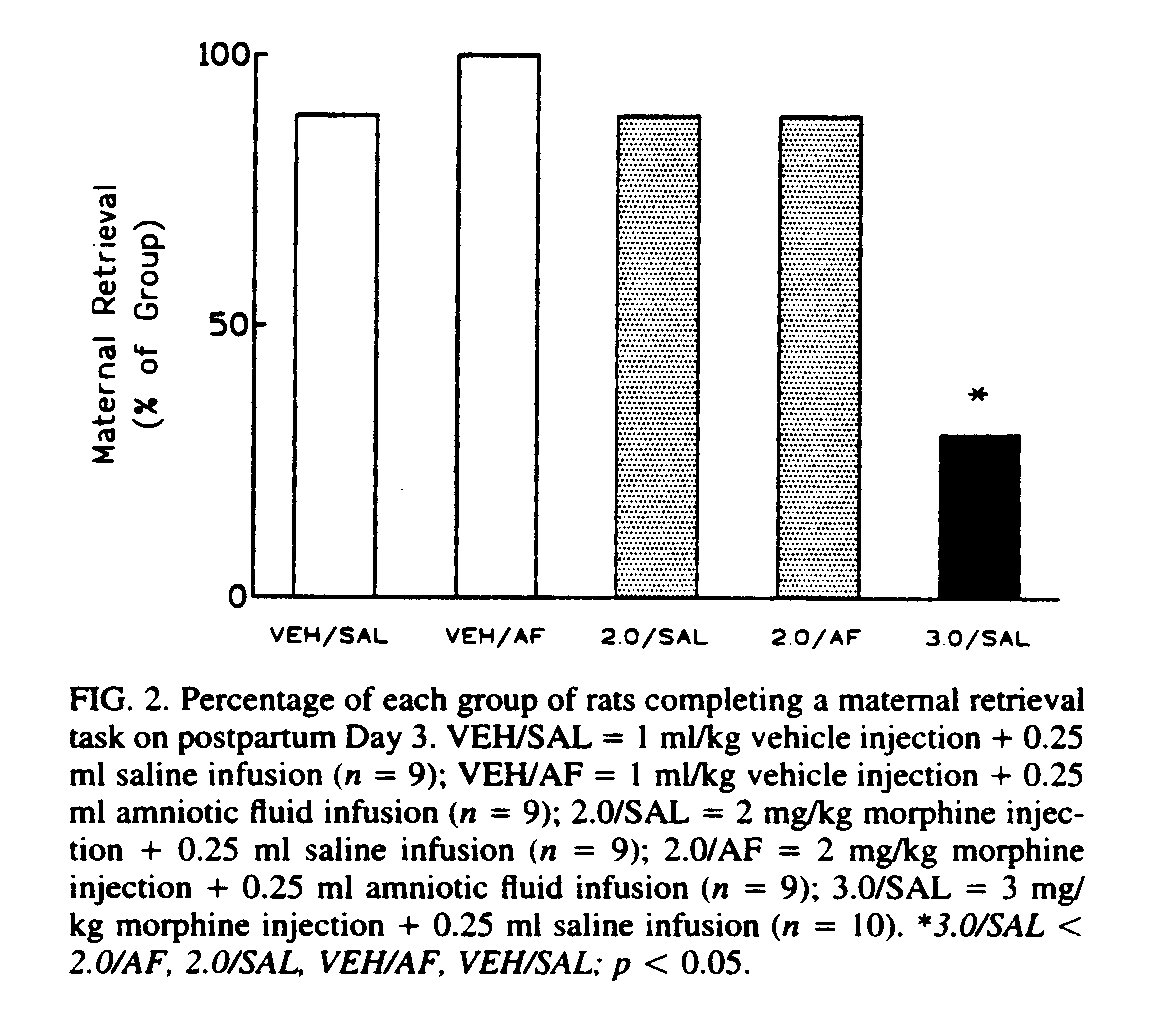
The results of the maternal behavior tests are presented in Fig. 2.
All the rats in the vehicle + amniotic-fluid group completed the retrieval task within the allotted time. One rat of nine failed to complete the task in both of the groups treated with 2 mg/kg morphine and in the vehicle + saline group. No significant differences were uncovered until comparisons were made with the 3 mg/kg-morphine group. Seven of the 10 rats in this group failed to retrieve their pups. Of the seven that failed to reach criterion, four retrieved 0 pups, one retrieved one pup, one retrieved three pups, and one retrieved four pups. The performance of the rats in this group differed significantly from that of each of the other groups (Fisher's Exact Probability Test, p£ 0.012). Consistent with previous observations (4,13,29), the disruption of maternal behavior appeared to occur without a decrease in general activity level. Morphine-treated rats were observed grooming themselves and exploring the cage, which sometimes even included stepping on pups without making any discernible effort to move them into the nest. No evidence was found of any effect of litter size on maternal behavior.
DISCUSSION
Testing for this experiment was conducted two days after de livery; therefore, testing assessed ongoing maternal behavior. Although testing for the onset of maternal behavior might have been more relevant, those results would have been confounded by the interference with delivery that would have been introduced by the necessary experimental manipulations. This shortcoming may be mitigated, however, by the finding that primiparous rats show a greater behavioral sensitivity to morphine in this circumstance than do multiparous rats (15), suggesting that the results obtained may be considered a reasonable approximation of what might be expected at the onset of the behavior.
Although this experiment was originally conceived as a 2 X 2 design, when it became apparent that it would be necessary to include a group of rats treated with 3 mg/kg morphine to expose the effects of the amniotic fluid, the possibility of adding a sixth group, one treated with 3 mg/kg morphine and receiving an orogastric infusion of amniotic fluid, was considered. Unfortunately, pilot data indicated that the analgesia experienced by such rats was beyond the scoring limits of our TFL assay. Four of the five rats in the pilot group did, however, successfully complete the pup-retrieval task, suggesting that the protective effect of amniotic-fluid ingestion on maternal behavior may generalize to doses beyond those used in the present experiment.
Just how or where morphine acts to interfere with maternal behavior and, consequently, why ingestion of amniotic fluid does not amplify this effect have yet to be determined. One possibility that has received some attention is that the disruption may result from the alteration of maternal body temperature (25) by morphine. Our findings are compatible with this possibility, as amniotic-fluid ingestion does not modify morphine-induced hyperthermia (1), but it is a possibility that preliminary evidence does not specifically support. It is also possible that the role of amniotic fluid (POEF) ingestion in enhancement of morphine-induced antinociception but not in morphine disruption of maternal retrieval is a function of location specificity, or even receptor specificity, of the morphine effects. Although morphine delivered to the preoptic area disrupts maternal retrieval (29), morphine delivered to the ventral tegmental area enhances the onset of maternal behavior in virgin female rats, and opiate antagonists delivered to the ventral tegmental area interfere with the maintenance of maternal behavior in postparturient rats (32,33).
In summary, the results of this experiment clearly show that ingestion
of amniotic fluid enhanced morphine-induced antinociception without potentiating
morphine-disruption of maternal behavior. These results offer an explanation
for why the action of the already elevated level of opioid is enhanced,
rather than the level itself being further increased, at parturition (16).
ACKNOWLEDGEMENT
This research was supported by NSF grant BNS 91-23748. awarded to M.B.K.
FOOTNOTE
REFERENCES