Brain Research 743 (1996) 184-201
Research report
Opioid stimulation
in the ventral tegmental area facilitates the onset of maternal behavior
in rats
Alexis C. Thompson*,
Mark B. Kristal
Behavioral Neuroscience Program,
Department of Psychology, State University of New York at Buffalo, Buffalo
NY 14260 USA
Accepted 20 August 1996
Abstract
This research investigated the effect of an increase or decrease in
opioid activity in the ventral tegmental area (VTA) on the onset of maternal
behavior in rats. In Experiment 1, the latency to show maternal behavior
toward foster rat pups (sensitization latency) was determined in maternally
naive female rats given either nothing or a unilateral intra-VTA injection
of morphine sulfate (MS) (0.0, 0.01, 0.03, 0.1 or 0.3 µg), on the
first three days of a 10-day period of constant exposure to pups. Rats
treated with 0.03 µg MS had significantly shorter sensitization latencies
than did rats treated with 0.0 µg MS, 0.01 µg MS, or receiving
no treatment (higher doses of morphine produced intermediate results).
The facilitating effect of intra-VTA MS on the onset of maternal behavior
was blocked by pretreatment with naltrexone hydrochloride and was found
to have a specific site of action in the VTA (MS injections dorsal to the
VTA were ineffective). In Experiment 2, sensitization latencies were determined
in periparturitional rats given a bilateral intra-VTA injection of either
the opioid antagonist naltrexone methobromide (quaternary naltrexone),
its vehicle, a sham injection, or left untreated 40 min after delivery
of the last pup. The mothers' own pups were removed at delivery; mothers
were nonmaternal at the time of testing. Quaternary naltrexone treatment
produced significantly slower sensitization to foster pups than did control
conditions. Total activity and pup-directed activity did not differ significantly
with treatment. The results demonstrate that increased opioid activity
in the VTA facilitates the onset of maternal behavior in inexperienced
nonpregnant female rats, and decreased opioid activity in the VTA disrupts
the rapid onset of maternal behavior at parturition.
Keywords: Maternal behavior; Opioid; Mesolimbic; Ventral tegmental
area; Naltrexone methobromide; Morphine; Rat
1. Introduction
The onset of maternal behavior in the rat, in the natural context of
parturition, is abrupt, rapid, and easily quantifiable by the appearance
of pup retrieval, pup grooming, and crouching over pups [13,61,82].
Maternal behavior can also be elicited by the presence of rat pups independent
of the circumstances of parturition in, for instance, virgin females, males,
and juveniles [60,82]. In these
nonparturitional cases, the maternal behavior exhibited is similar to postpartum
maternal behavior; pup retrieval, pup licking, and crouching over pups
is present, differing mainly in the duration of pup exposure required to
elicit the behavior (h or days vs. min) [15].
These observations regarding the onset of maternal behavior have led to
the idea that maternal behavior is induced primarily by stimuli associated
with parturition and emanating from the pups, and is facilitated by the
endocrine and neurochemical changes associated with pregnancy and parturition
[52,68,70].
Endogenous opioids have been considered
as one possible candidate mediating the rapid onset of maternal behavior
at parturition [38]. Plasma and
brain levels of endogenous opioids increase over the course of pregnancy,
peak during parturition, and then decline to pre-pregnancy levels during
lactation [58,80]. These changes
in endogenous opioids are consistent with at least one behavioral change
during parturition: pain threshold is greatly elevated in the periparturitional
period by an opioid-mediated mechanism [43].
Only a few studies have assessed the role of opioids in the onset and maintenance
of maternal behavior, and the findings are mixed. Given systemically, both
opioid agonists and antagonists have been shown to reduce or delay maternal
responding [5,20,40,50,63]. Given
centrally, opioid agonists injected i.c.v. have been shown to facilitate
the onset of maternal behavior in sheep [35,37] whereas
injections directly into the medial preoptic area have been shown to block
ongoing maternal behavior in the rat [45,46,62].
Finally, Kristal et al. [43]
have found some measures of maternal behavior to be highly correlated with
increased opioid activity during parturition.
One explanation for these apparently
contradictory results may lie in the distribution and action of endogenous
opioids. Endogenous opioids are found at many levels of the nervous system
and the responses elicited by opiate administrations at different brain
sites frequently oppose each other [30,31].
Systemically administered opioid agonists and antagonists may produce a
variety of effects depending upon where in the nervous system they act,
or which opioid receptor field contributes most to the final behavior.
A better approach to studying opioid effects on maternal behavior would
be to apply opioid agonists and antagonists to specific brain areas thought
to be part of the maternal behavior neural substrate and also known to
contain an opioid receptor field. Bridges [45, 46,62] has
shown that increased opioids in the medial preoptic area inhibit maternal
responsiveness; the medial preoptic is an area widely accepted to be crucial
to the mediation of maternal behavior [52]
. The experiments presented here investigate the actions of endogenous
opioids in the ventral tegmental area (VTA) on the maternal responsiveness
[76]. The VTA, like the medial
preoptic area, is a crucial component of the "maternal neural substrate"
[18,22-25,54], but in contrast
to the medial preoptic area, the VTA is also a principal site at which
opioids act to stimulate motivated behavior [2,30,31].
Experiments 1A and 1B were designed
to test the hypothesis that increased opioid activity in the VTA facilitates
the onset of maternal responsiveness in a dose-dependent fashion. First,
the effect of injecting several doses of the opioid agonist morphine sulfate
(MS) into the VTA on the latency to onset of maternal behavior was determined
(Exp. 1A). Second, the pharmacological specificity of the action of morphine
on opioid receptors was directly assessed by treating some rats with the
opioid-receptor antagonist naltrexone hydrochloride, systemically, prior
to the intra-VTA injection of morphine (Exp. 1B). If the action of morphine
on maternal behavior in Exp. 1A is through the opioid receptor, then administering
an opioid-receptor antagonist should block the facilitating effect of morphine
on the onset of maternal responsiveness. Finally, the anatomical specificity
of the observed results was assessed in some rats by injecting morphine
dorsal to the VTA. If the effect of morphine on maternal behavior observed
in Exp. 1A is specific to its action in the VTA, then morphine injected
dorsal to the VTA should have no effect on the latency to onset of maternal
responsiveness (Exp. 1B).
Experiment 2 was designed to determine
if the findings of Exp. 1 could be applied to the induction of maternal
behavior in a more natural context. Bilateral intra-VTA injections of the
quaternary opioid antagonist naltrexone methobromide, or its vehicle, were
administered to newly parturient rats that had been prevented from developing
maternal responsiveness during parturition, and the consequent latency
to show full maternal behavior was assessed. Control rats were expected
to show rapid development of maternal responsiveness because the periparturitional
period provides the natural context for that rapid development [4,55].
We hypothesized that VTA opioid activity is necessary for the development
of maternal behavior; therefore we would expect the experimental dams,
those with blocked VTA opioid activity, to show a delay in the development
of full maternal behavior (i.e., longer latencies to onset of maternal
responsiveness).
2. Materials and methods: General
2.1. Subjects
Subjects were nulliparous female
Long-Evans (hooded) rats, 3 to 5 months old, raised in our laboratory from
Harlan Sprague-Dawley (Blue Spruce Farms) stock.
Rats were maintained in a controlled
environment with an ambient temperature of 22 ± 1 °
C, a relative humidity of about 50%, and a 14 h on/10 h off light cycle
(lights on at 05.00 h, EST). During lights off, the room was illuminated
by a red 60-W bulb. Rats were housed individually in 24.5 X
18 X
18-cm suspended wire-mesh cages except during the testing period when they
were housed in specialized observation cages (see below). Food (Agway Prolab
Rat/Mouse/Hamster Formula 3000) and water were available ad lib
throughout all experiments.
Each potential subject was screened
for spontaneous retrieval of pups or cannibalism by observing her initial
response to 2 foster pups [70].
Rats observed to retrieve or bite the pups in a single 15-min period (approximately
5% of those tested) were removed from the pool of subjects. (Pup-naive
nulliparae that show spontaneous retrieval or cannibalism are rare enough
not to be distributed evenly across groups, and acquire different experience
with pups than do the more common pup-avoiders [27,57].)
2.2. Stereotaxic surgery
Subjects, except those assigned to
no-treatment control groups, were implanted with permanent indwelling guide
cannulae through which drugs could be injected directly into the VTA. Guide
cannulae consisting of either a 22-ga stainless steel tube (Plastic Products,
Exps. 1A and 1B) or a 23-ga stainless steel tube (Small Parts, Inc., Exp.
2), were aimed at an area 1 mm dorsal to the posterior aspect of the VTA
(A-P: - 3.8, L: ± 0.6, and D-V: - 9.5 [56]
). It was expected that these coordinates would place the tip of the microinjection
cannula into the area of the VTA previously shown to yield the best behavioral
response to opioid microinjections [2,59].
In Exps. 1A and 1B, guide cannulae were implanted unilaterally, alternating
between left and right placements. In Exp. 2, cannulae were implanted bilaterally
approximately 1 mm apart. During surgery, rats were anesthetized with pentobarbital
sodium (40 mg/kg, i.p.) after pretreatment with atropine sulfate (4 mg/kg,
s.c.) to suppress mucus secretion. At the end of surgery, the cannulae
were closed by inserting stylets, and the wound was covered with a sulfa-urea
antiseptic powder (Sulf-U-Dex). A systemic antibiotic (Combiotic, 0.5 ml,
i.m.) was given immediately after surgery and on postoperative Day 3 as
a prophylactic measure. Rats were given an 8- to 14-day recovery period
before testing.
2.3. Stimulus pups
In all experiments, stimulus pups
were foster rat pups obtained from the litters of donors maintained in
a separate colony room. Exposing maternally naive adults to foster pups
for a prolonged period (concaveation leading to maternal sensitization)
necessitates replacing the pups at regular intervals to insure continued
stimulus quality and survival of the pups because the subjects neither
lactate nor necessarily behave maternally [60,70]
In these experiments, pups were exchanged approximately every 12 h with
pups that had been fed and cared for by their natural mother (donor) over
the preceding 12 h (at minimum) period. Pup exchange occurred at the start
of each 12 h observation period.
2.4. Apparatus
Observation cages, in which experimental
rats were housed during the testing period, were 10-gal glass or plastic
aquaria. Mirrors were positioned behind each cage to facilitate observation
by the experimenter. Rats were prevented from seeing one another by cardboard
partitions placed between the cages.
All behavior observations were made
over closed-circuit television equipped with a remotely controlled zoom
lens and pan-tilt gimbal. The zoom and pan-tilt controls, a recorder, and
a monitor were housed in a room adjacent to the observation room.
A computer (DOS-based 286 micro-processor,
16 MHz) was used as a continuous recorder (I/O sampling every 8 s) with
the aid of a program (MONITOR2) designed to record and then extract frequency,
onset, and duration data for each of several behaviors during a testing
period [73,74]. Observations
were limited to 1 to 2 rats at a time to maximize visibility.
2.5. Drug injections
Injection of drug into the VTA was
accomplished by inserting a 28-ga internal cannula into the guide cannula
and injecting into brain tissue a 0.5 ml
solution containing the appropriate dose of drug. A micro-infusion pump
(Harvard, Model 944) was used to inject at a rate of 1 m
l/min. The internal cannula extended 1 mm beyond the guide cannula and
was left in place for 30 s after the drug injection was completed. The
experimenter was blind to the drug condition until the end of the sensitization/testing
period.
2.6. Behavior observations
During the testing period, two 15-min
behavior observations were made each day: once during the lights-on phase
(between 09.00 h and 11.00 h) and once during the lights-off phase (between
21.00 h and 24.00 h). In Exp. 2, additional behavior observations were
made during the first 12 h of the sensitization period.
2.6.1. Maternal-behavior tests
During the behavior observations,
the presence or absence of full maternal behavior was noted. At the start
of each observation period, 4 freshly fed and cared for pups were scattered
about the observation cage and the presence of pup retrieval to the nest,
pup licking, and crouching over the pups was scored. When a rat showed
all three of these pup-directed behaviors consistently, in two consecutive
observation periods, she was considered fully maternal. The dependent measure
derived from the maternal-behavior test was latency, in days (Exps. 1A
and 1B) or hours (Exp. 2), to show full maternal behavior. Each rat was
assigned the latency that corresponded to the first observation period
of the two consecutive observation periods in which she showed full maternal
behavior. The protocol used to score full maternal behavior corresponds
to the standard maternal-behavior test protocol used during sensitization
to rat pups [60,70,82]. The latency
to show full maternal behavior (sensitization latency) was used to test
the primary hypothesis that an increase in opioid activity in the VTA facilitates
the onset of maternal responsiveness.
In addition to measuring sensitization
latency, the latency to engage just in retrieving, which is highly correlated
with the other maternal behaviors, was indirectly assessed by noting whether
or not the test rat had gathered her foster pups into her nest between
behavioral observations (latency to show pup-grouping). Such pup grouping
would be an indication of some maternal responsiveness even in rats that
failed to meet the criteria for full maternal behavior.
2.6.2. Other behavioral measures
Other behaviors were also measured
during each 15-min observation period to test secondary hypotheses about
drug-induced changes in pup-directed behavior and motor activity. These
behavior measures assessed duration of (a) pup-directed behavior (sniffing,
licking, retrieving, and crouching over the pups); (b) object-directed
behavior (sniffing, licking, carrying, and gnawing the other stimulus objects;
Exps. 1A and 1B only); (c) self-directed behavior (grooming and eating);
(d) duration of nest-related activity (nest building and nest rearrangement
within the nest quadrant); (e) forward locomotion; and (f) resting.
2.7. Statistical analysis
2.7.1. Latency data
The sensitization/testing period
was terminated after 9.5 days, thereby truncating the latency data for
nonresponders (Exps. 1A and 1B). Therefore, medians were used to express
group latencies (sensitization latency and latency to show pup-grouping),
and nonparametric statistical tests were used to assess the significance
of group differences. The test statistic was the Kruskal-Wallis One-way
Analysis of Variance by Ranks (KW test) corrected for tied ranks [64].
A multiple-comparisons test [64]
was used to probe significant group differences identified by the KW test.
The number of pairwise comparisons necessary to identify differences among
experimental and control groups was minimized by first comparing control
groups, and, when found not to differ from each other (as expected), using
only the single most appropriate control group.
2.7.2. Duration data
The durations of self-directed, pup-directed,
object-directed, and nest-directed behaviors, forward locomotion, and resting
were determined during each 15-min observation. However, only a sample
of the observation periods obtained for each rat was used to assess, statistically,
the effect of intra-VTA drug treatment on responsiveness to pups and on
general level of activity. Observation periods were chosen so as to maximize
the chance of detecting either acute (immediately after treatment) or enduring
(long after treatment) differences in the behavior of rats before, during,
and after becoming maternal. We hoped that such information would complement
the interpretation of treatment effects on onset of maternal behavior,
for example, that specific treatments appear to enhance the onset of maternal
behavior because they result in increased contact with pups. The initial
inspection of the data revealed that rats tended to spend most of the observation
period engaged in only one or two of the behavior categories (resting,
nest-directed, forward locomotion, or self-directed behavior). Duration
scores within each behavior category were not normally distributed, but
clustered at each end of the duration interval, thereby compromising a
multivariate statistical analysis on all dependent variables [72].
Therefore, a more limited data analysis was performed to assess whether
significant differences in (a) the amount of pup contact (total time spent
in pup-directed behavior) and (b) amount of total activity (total time
spent in any active behavior), existed between treatment groups. The effects
of treatment on pup contact and total activity were assessed separately
using 1-way ANOVAs and adjusting a
to control for multiple comparisons.
2.8. Histological examination
Each cannulated rat was injected
with pentobarbital sodium (1 ml, i.p.) and its brain removed, frozen (-20
°
C), and cut in 40-m
sections. Methyl blue dye (0.5 ml)
was injected through an internal cannula just before the pentobarbital
injection to facilitate visual localization of the cannula tip during sectioning.
Every fourth section cut from the area around the cannula site was saved
and stained with cresyl violet. Cannula placement was verified by inspection
and comparison with the stereotaxic atlas of Pellegrino, Pellegrino, and
Cushman [56].
3. Experiment 1A
The effect of each of five doses
of intra-VTA morphine sulfate on the sensitization latency was examined.
Intra-VTA morphine was expected to decrease, in a dose-dependent fashion,
the latency for nulliparous, maternally naive female rats to show maternal
behavior (i.e., expected to facilitate the onset of maternal behavior).
The morphine dose ranged from 0.01 m
g to 0.3 m
g per injection and was intended to be less than that needed to produce
large increases in locomotor activity [29].
In a pilot study in which rats were given the highest dose of morphine
(0.3 m
g) for 3 consecutive days, we observed no marked increase in locomotor
activity and no overt behavioral/physiological symptoms of withdrawal upon
drug cessation.
3.1. Method
3.1.1. Subjects
Sixty nulliparous nonpregnant (virgin)
rats were randomly and evenly distributed among six groups: four doses
of morphine (0.01, 0.03, 0.1, and 0.3 mg);
a vehicle-injected control group (0.0 µg morphine); and a no-surgery
control group (no treatment).
3.1.2. Apparatus
In these experiments, four stimulus
objects other than rat pups were also presented to the experimental rats
at the same time as the stimulus pups, to provide alternative novel stimuli.
The four stimulus objects were an eyedropper bulb (1.25-cm long X
1.25-cm diameter at its widest point); an acrylic pompom (2.5 cm, Darice);
a hamster chew stick (5 X
1.25 X
.75 cm, V.I.P.); and a plastic ring (2.5-cm diameter, Dritz). Eyedropper
bulbs have been used by other experimenters for roughly the same purpose
[27].
The observation cage was 51 X
25 X
31 cm, covered by a wire-mesh top. The cage floor was subdivided into quadrants
by 3-cm-high Plexiglas dividers. The height of the dividers was sufficient
to prevent pups from crawling out of the quadrant, and thereby initiating
contact with the adult, without impeding the movement of the adult rat
around the cage [53]. The cage
floor was covered with 1 - 2 cm of standard wood shavings (Aspen laboratory-grade
shavings).
3.1.3. Drugs
Morphine sulfate was mixed in 0.9%
sterile saline and injected once each day during the first 3 days of the
sensitization period (Day 0, 1, and 2).
3.1.4. Experimental Procedures
Each rat was moved into an observation
cage one day before the start of sensitization, to habituate to the cage
environment. One hour before the morning observation on each day of sensitization,
stimulus pups and other stimulus objects were removed, stage of estrous
cycle of the test rat was determined by vaginal smear, and on the first
three days of the testing period, drug was administered. For each rat,
these procedures took about 3-5 min. The vaginal smears were taken and
drug was injected in a room adjacent to the observation room. Immediately
after drug injection, rats were returned to their observation cages; food,
water and fresh bedding were added if necessary.
One hour later, fresh stimulus pups
and stimulus objects were placed in the test cage, and the observation
period was begun. Stimulus pups and objects were "scattered" around the
observation cage by placing two pups and two objects in the quadrant diagonal
to the rat's resting quadrant and one pup and one object in each of the
quadrants adjacent to the resting quadrant. For the dark-phase test, pups
were switched but other manipulations, and the 1-h delay, were omitted.
In addition to testing for maternal
behavior, as described above, we assessed the durations of pup-directed
behavior and general activity during the morning observation on Day 2 (1
h after the last daily drug injection), the morning observation on Day
5 (72 h after the last daily drug injection), and during the first observation
in which full maternal behavior was observed.
3.2. Results and discussion
During the experiment, four rats
failed to complete the entire testing sequence (e.g., had unusable indwelling
cannulae) and were dropped from all subsequent analyses. At the end of
the experiment, histological examination showed that cannula placement
in 35 of the remaining 47 cannulated rats fell within the VTA (Fig. 1).
In these rats, the cannula placement fell within the area bounded anteriorly
by the appearance of the interpeduncular nucleus, posteriorly by the appearance
of the pons, medially by the interpeduncular nucleus, laterally by the
lateral lemniscus, ventrally by the base of the brain and dorsally by the
dorsal extent of the interpeduncular nucleus. In 7 of the 12 rats in which
cannulae were judged to have missed the VTA, placement was ventral to the
VTA, piercing through the base of the brain (2 treated with 0.3 µg
morphine, 1 treated with 0.1 µg morphine, 1 treated with 0.03 µg
morphine, 2 treated with 0.01 µg morphine, and 1 treated with 0.0
µg morphine). The five remaining "misses" were anterior (1 treated
with 0.3 µg morphine and 1 treated with 0.03 µg morphine) or
dorsal (1 treated with 0.1 µg morphine, 1 treated with 0.03 µg
morphine, and 1 treated with 0.01 µg morphine). Data from these "missed"
cannula placements were excluded from group statistical analyses. During
this experiment, all rats continued to show normal estrous cyclicity, regardless
of drug treatment; no significant correlation between estrous cycle stage
and the onset of maternal behavior was observed.
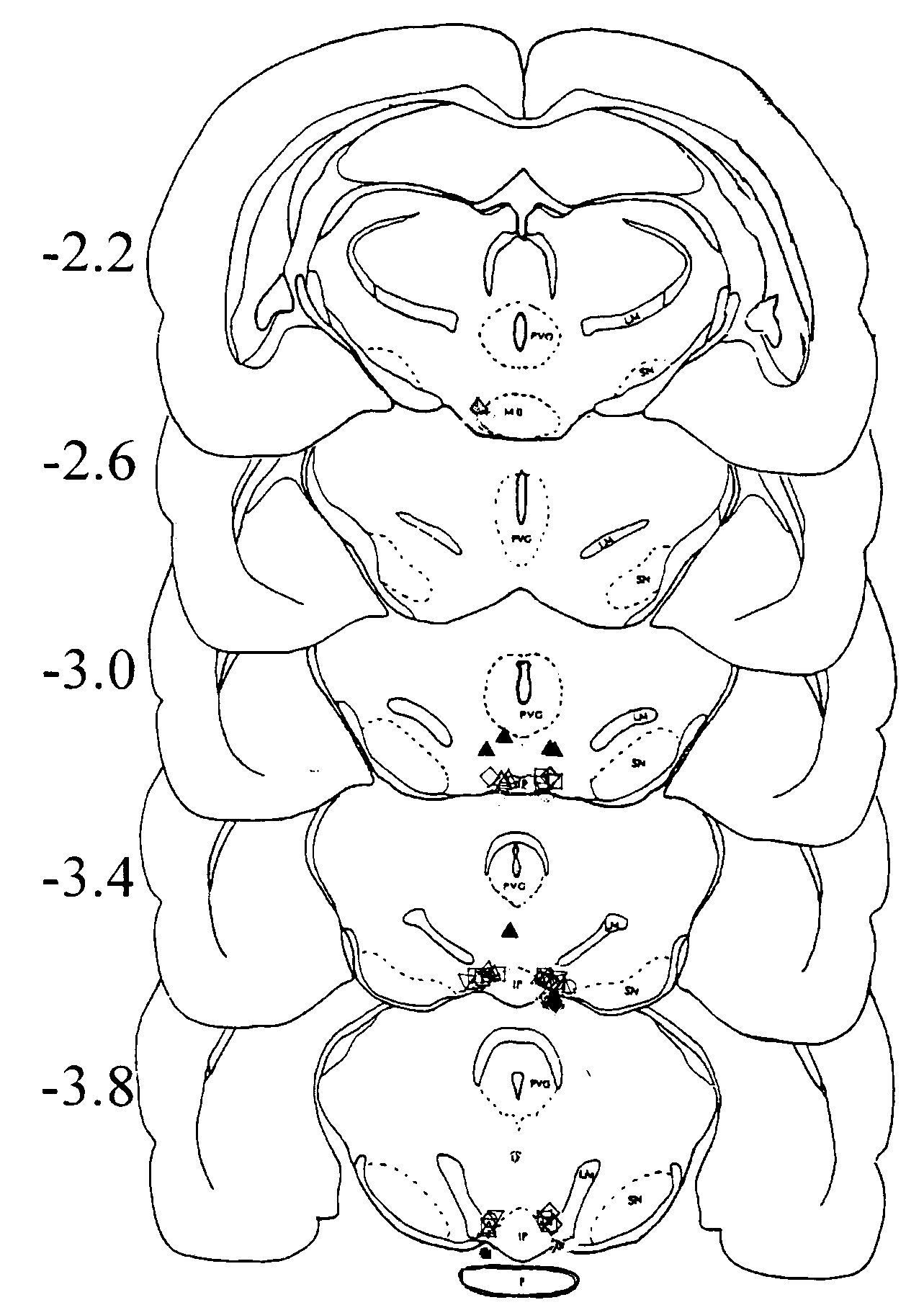
Fig. 1. Schematic representation
of the location of cannula tips in Expts. 1A and 1 B. Open symbol, 'hits'
Expt. 1A, data used in the group statistics; gray symbol 'misses' Expt.1A,
data excluded from the group statistics. open square, 0.0 mg
morphine; open circle, 0.01 mg
morphine; open up triangle, 0.03 mg
morphine; open down triangle, 0.1 mg
morphine; open diamond, 0.3 mg
morphine; up filled triangle, dorsal placements in Expt. 1B (0.03 mg
morphine). IP, interpeduncular nucleus; LM, medial lemniscus; MB, mammillary
bodies; PVG, periventricular gray substance; P, pons; SN, substantia nigra.
Diagram after Pellegrino, et al. [56].
3.2.1. Latency to the onset of full
maternal behavior (sensitization latency)
The median sensitization latencies
of each group are reported in Table 1. A KW test comparing the sensitization
latencies in each group was statistically significant (KW(5) = 17.81,
P = 0.003). A subsequent multiple pairwise comparison probe of drug-treated
rats (excluding the no-treatment group) revealed that rats treated with
0.03 µg MS had significantly shorter sensitization latencies (median
= 5.5 days) than did rats treated either with 0.01 µg MS or 0.0 µg
MS (median = 10 days for each group; P < 0.05). The sensitization
latencies in rats treated either with 0.1 µg MS (median = 8.5 days)
or 0.3 µg MS (median = 10 days) were not significantly different
from any other group (P > 0.10). As expected, no statistically significant
differences were found between vehicle-injected rats (0.0 µg MS)
and those in the no-treatment group (KW(1) = 0.07, P = 0.80).
Table 1.
Median sensitization latency to show full maternal behavior and
median latency to show pup grouping in Expt. 1A.
|
Treatment
|
n
|
Median sensitization latency
(days)
|
Median latency to show pup
roupingc (days)
|
|
Intra-VTA morphine
|
|
|
|
|
0.3 µg
|
7
|
10.0
|
9.0
|
|
0.1 µg
|
8
|
8.5
|
8.0
|
|
0.03 µg
|
7
|
5.5b
|
4.5
|
|
0.01 µg
|
5
|
10.0
|
10.0
|
|
0.00 µg
|
8
|
10.0
|
10.0
|
|
No treatmenta
|
9
|
10.0
|
8.0
|
aNot used in the statistical analysis of
the differences between specific drug doses. No Treatment and 0.0 µg
control groups did not differ significantly from each other in either sensitization
latency or latency to show pup grouping (P > 0.05).
bSignificantly different from 0.0 µg and 0.01 µg MS
groups (P < 0.05).
cThe overall comparison of the drug treated groups showed a statistically
significant difference (P < 0.05). |
The intermediate effectiveness of
MS at the higher doses is suggested by the proportion of rats showing full
maternal behavior at different points during the testing period (Fig. 2).
Whereas only 22-25% of the control rats (no-treatment and 0.0 µg
MS) showed full maternal behavior during the 10-day test period, 100% of
the rats treated with 0.03 µg MS, 75% of the rats treated with 0.1
µg MS, and 43% of the rats treated with 0.3 µg MS showed full
maternal behavior during the sensitization period. Nonparametric analyses
[14] revealed
that the proportions of the 0.03 and 0.1 µg MS groups showing full
maternal behavior during the testing period were significantly greater
than that of the 0.0 µg MS group (overall X2(4)
= 13.7; P < 0.01; 0.03 µg vs. 0.0 µg: X2(1)
= 8.7, P < 0.005; 0.1 µg vs. 0.0 µg: X2(1)
= 4.0, P < 0.05; 0.3 µg vs. 0.0 µg: X2(1)
= 0.5, > 0.05).
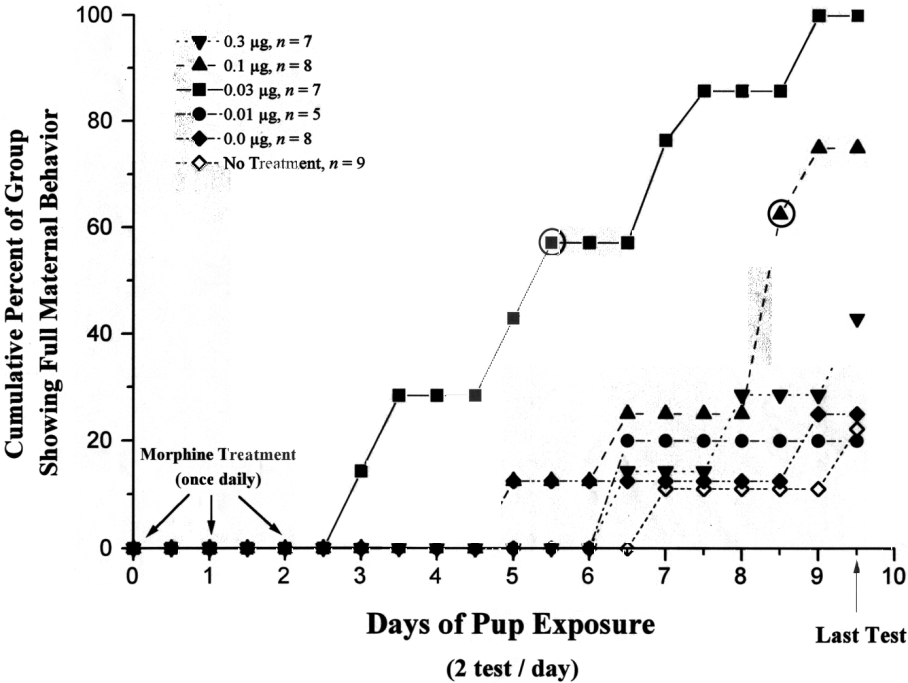
Fig. 2. Cumulative percent of group showing full maternal
behavior at each test over the 9.5-day sensitization/testing period. Nonmaternal
nulliparae were given unilateral intra-VTA injections of morphine (0.0,
0.01 0.03, 0.1, or 0.3 µg) or no treatment 1 h before the morning
test on Days 0, 1, and 2. The median latency of the 0.03 µg and 0.1
µg morphine groups is indicated by an open circle . Remaining groups
were assigned a median latency of 10 (0.5 day longer than the longest possible
median) because fewer than half of the rats in the these groups showed
full maternal behavior during the sensitization period. The proportion
of rats showing full maternal behavior during the pup-exposure period was
significantly greater in the 0.03 µg and 0.1 µg morphine groups
than in the 0.0 µg morphine group (P < 0.05).
The sensitization latencies in rats
with cannula placements outside the VTA were similar to those of controls.
Three rats injected with 0.03 µg MS into areas dorsal (n =
1), anterior (n = 1), and ventral (n = 1) to the VTA, showed
sensitization latencies similar to control values (10 days each) rather
than similar to those of rats treated with 0.03 µg MS in the VTA
(median5.5 days).
3.2.2. Latency to show pup grouping
A test comparing the median latencies
to show pup grouping in each treatment group (Table 1) revealed an overall
statistically significant difference (KW (5) = 11.75, P = 0.04).
However, pairwise comparisons of the five drug-treated groups did not find
any reliable group differences at P < 0.05. Although the extraneous
objects (chew stick, pompom, eyedropper bulb, plastic ring) were occasionally
manipulated and mouthed by the subjects, no reliable patterns of behavior
(e.g., relocation, retrieving, grouping) directed toward these objects
was ever detected.
3.2.3. Behavior during the observation
period
One-way ANOVA was used to test group
differences in pup-directed behaviors and total active behavior at each
observation (Day 2, Day 5, and during full maternal behavior), yielding
a total of six 1-way ANOVAs. A modified a
level was used so that statistical significance in any one test was achieved
if a
was less than 0.008 (overall for 6 ANOVAs, total a
= 0.048).
Day 2 morning observations.
The time spent (mean ± S.E.M.) in each behavior category during
the 15-min observation on the morning of Day 2 (1 h after drug treatment)
is depicted in Fig. 3. Overall, rats in all groups spent most of their
time engaged in an active behavior, with mean total activity scores ranging
from 560.6 ± 120.5 s (in the 0.03 µg MS group) to 883.4 ±
9.6 s (in the 0.1 µg MS group). Very little time was spent in pup-directed
behavior, typically less than 30 s. No significant differences in pup-directed
behavior or total active behavior were found among groups by 1-way ANOVA
(pup-directed behavior: F5,38 <
1; total active behavior: F5,38 =
3.45, P > 0.008). A more limited comparison between the group that
showed the most rapid onset of maternal behavior (0.03 µg morphine)
and the controls also revealed no differences in either pup-directed or
total active behavior (Ps > 0.05).
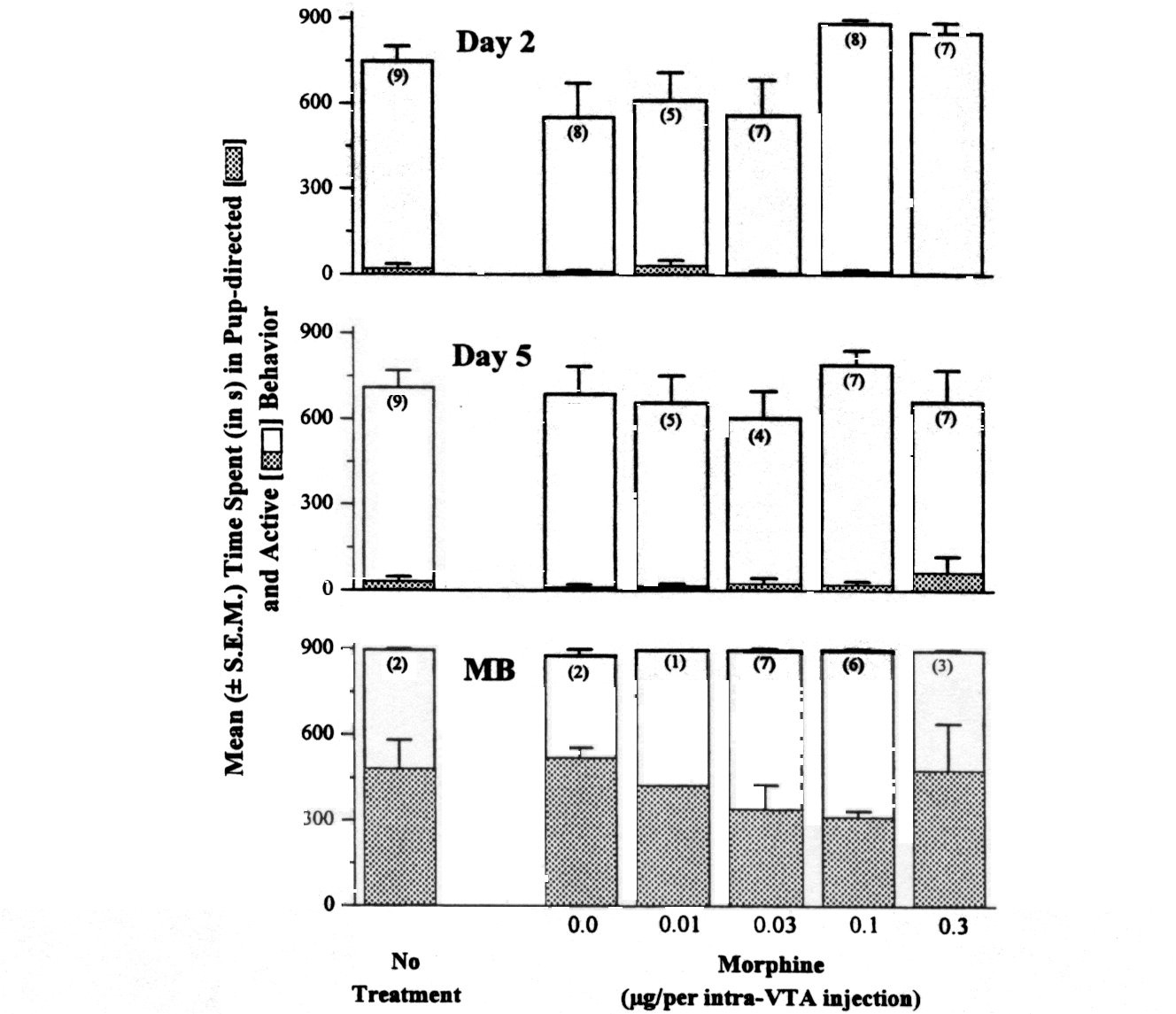
Fig. 3. Mean ± S.E.M. time spent ( in
s) in pup-directed or active behavior during the 15-min test on the morning
of Days 2 and 5, and during the test in which full maternal behavior was
observed (MB). Nonmaternal nulliparae were treated with either intra-VTA
morphine (0.0, 0.01, 0.03, 0.1, 0.3 µg) or no treatment 1 h before
the morning test on Days 0, 1, and 2. The number in parentheses represents
the number of rats observed on that test day (rats showing maternal behavior
before Day 5 were not among those tested on Day 5; rats not ever showing
full maternal behavior were not tested on Day MB). No significant differences
among groups in pup-directed behavior or active behavior were found on
any day.
Day 5 morning observation.
The time (mean ± S.E.M.) spent in each behavior category during
the 15-min observation on the morning of Day 5 (3 days after the last drug
injection) is reported in Fig. 3. The pattern of data is similar to that
obtained in the Day 2 observation. No differences in either pup-directed
behavior (F5,33 <
1) or total active behavior (F5,33
< 1) were found. Note that five rats (three from the 0.03 µg MS
group, one from the 0.1 µg MS group, and one from the 0.0 µg
group) were not observed during this observation as they had already shown
full maternal behavior by Day 5. None of the remaining rats was showing
full maternal behavior during the Day 5 observation, although approximately
55% (21/39) of these rats would show full maternal behavior over the next
4 days.
Observation after the onset of
full maternal behavior. The time (mean ± S.E.M.) spent in each
behavior category during the 15-min observation in which each rat showed
full maternal behavior for the first time is reported in Fig. 3. In contrast
to the rats on the previous observation days (Day 2 and Day 5), those showing
full maternal behavior spent a much greater proportion of time in pup-directed
behavior (approximately 360-480 of 900 s). No significant differences were
found in the 1-way ANOVA that compared pup-directed behavior among the
rats receiving the three highest drug doses (0.3, 0.1, and 0.03 µg
MS) and controls (no treatment and saline combined) (F3,16
=
1.51, P = 0.25).
The results of Exp. 1A support the
hypothesis that opioid activity in the VTA facilitates the onset of maternal
responsiveness, by showing that increased opiate activity in the VTA (by
injection of morphine) decreased the maternal sensitization latency in
a dose-dependent fashion. An inverted U-shaped pattern of dose-responsiveness
was obtained in which the effectiveness of MS was reduced as the dose increased,
presumably as a result of an increase in a competitive response(s), reduced
receptor selectivity, or both. The first observation period was intended
to evaluate the possibility of the appearance of a competitive response.
This observation was made 1h after the third MS administration, that period
when the effect of MS should have been maximal: Acute microinjection of
MS into VTA increases activity over several hours, peaking at about the
end of the first hour [26,29],
and
repeated MS administration enhances this effect [79].
No
unusual behaviors were observed by casual inspection and no significant
group differences in total activity were found during the first observation
period. Although the activity level of the two highest drug dose groups
may have been maximal in that the rats remained active over the entire
15 min sampling period (Fig 2). It is possible that had the observation
period been longer, it would have revealed significantly higher total activity
levels in these two highest dose groups relative to the remaining treatment
groups. Such a finding would then suggest that an increase in general activity
may have competed with the development of maternal behavior. Changes in
receptor selectivity seem less likely. Certainly the doses used here were
not so high as to induce non-opioid receptor-mediated effects. The doses
we used were well below those typically used in studies of opioid-induced
locomotor activation [26,29].
However, the VTA contains multiple opioid receptor types [47]
and so it is possible that the proportional stimulation of these receptor
types varied over the range of MS doses used here. In the absence of additional
data (i.e. longer observation periods or more selective opioid-receptor
agonists), we can only speculate about the reduced efficacy of the higher
drug doses.
No rat showed full maternal behavior
(or pup grouping) during the 3-day period of drug injections, therefore
our results do not reflect an acute drug-bound change in the expression
of behavior, but rather a drug-induced increase in the induction of maternal
behavior toward pups.
Fewer than half of the rats in controls
groups developed full maternal behavior during the 10 day testing period.
Large variations in sensitization latencies between laboratories have been
reported and ascribed to differences in strain [28,67] and
housing environment [75]. In
this case , it seems probable that the steps we took to reduce pup-initiated
contact (the addition of floor dividers) increased the co-habitation time
needed to induce full maternal behavior rather than blocked the development
of maternal behavior. Whereas it is possible that these rats may
have never shown maternal behavior, the overwhelming preponderance of data
in this field suggests that they would eventually do so.
Finally, the ancillary behaviors
of rats in that dose group with the shortest sensitization latencies (0.03
µg MS) did not differ from those of control rats. In addition, the
reduced efficacy of higher MS doses would seem to rule out the possibility
that drug-induced increases in the period of activation increased incidental
pup contact and reduced sensitization latencies; higher doses of MS treatment
would in fact be predicted to produced faster development of maternal
behavior. Therefore, no specific differences in general behavior or pup-directed
behavior were observed that might account for the facilitated onset of
maternal responsiveness among the rats receiving 0.03 µg MS.
4. Experiment 1B
The conclusion reached in Exp. 1A,
that morphine sulfate injected into the VTA facilitates the onset of maternal
behavior, rests on either or both of two key assumptions about the action
of the MS once it is injected into the VTA. Specifically that (a) the effect
of intra-VTA morphine on maternal responsiveness is the result of the action
on opioid receptors (receptor specificity), and (b) the effect of intra-VTA
morphine on maternal responsiveness is the result of the action of morphine
within the VTA (anatomical specificity). Experiment 1B tested these assumptions
by determining the sensitization latency in two groups of rats pretreated
systemically with the opioid antagonist naltrexone hydrochloride or vehicle
and receiving the most effective intra-VTA dose of morphine found in Exp.
1A (0.03 µg), and one group of rats pretreated systemically with
vehicle and receiving the most effective dose of morphine in a neuroanatomical
site dorsal to the VTA. We expected that either pretreatment with the antagonist
or injection into a site other than the VTA would prevent the facilitating
effects of morphine on the onset of maternal behavior, therefore reflecting
the receptor-specific and location-specific actions of intra-VTA morphine
injection.
4.1. Method
4.1.1. Subjects
Twenty-five virgin rats were used.
In 20, cannula placement was aimed at the VTA; in the remaining 5, the
cannula placement was aimed at a point 2mm dorsal to the VTA. Rats with
VTA placements were randomly assigned to one of two pretreatment conditions
(naltrexone hydrochloride: n = 10, or vehicle: n = 10); rats
with dorsal-to-VTA placement were pretreated only with vehicle (for comparison
to VTA/vehicle-treated rats).
4.1.2. Drugs
The naltrexone hydrochloride (1 mg/kg,
s.c.) was mixed in 0.9% sterile saline and injected 10 min before the intra-VTA
injection of morphine sulfate (0.03 µg). The dose of naltrexone hydrochloride
(1 mg/kg) was found in a pilot study to be effective in blocking the action
of intra-VTA MS (1 µg in 0.5 ml) on morphine-induced forward locomotor
activity.
One rat from the dorsal VTA placement
group was lost as the result of surgery and two rats from the vehicle-pretreatment
intra-VTA group did not complete the testing sequence after the integrity
of their indwelling cannula was compromised. Histological examination revealed
that cannula placements of 16 of the remaining 22 rats fell in the expected
area of the brain (Fig. 1). In 6 rats, the cannula placement that was intended
to hit the VTA fell outside the area of the VTA (4 from the naltrexone-pretreatment
group and 2 from the vehicle-pretreatment group). The missed cannula placements
were dorsal (n = 4), anterior (n = 1), or ventral (n
= 1) to the VTA. Data from the missed cannula placements were excluded
from group statistical analyses, except for one rat with a dorsal placement
that had also received pretreatment with vehicle. Her treatment was identical
to that of rats in the planned dorsal-to-VTA group and so her data were
added to that group.
4.2.1. Maternal behavior
Median sensitization latencies for
each group in Exp. 1B are reported in Table 2. As expected, pretreatment
with 1 mg/kg naltrexone hydrochloride blocked the facilitating effect of
0.03 µg MS in the VTA on sensitization latencies. Vehicle-pretreated
rats with cannulae in the VTA had significantly shorter sensitization latencies
(median = 6.5 days) than did naltrexone-pretreated rats (median = 10 days)
(KW(1) = 5.32, P = 0.02). The same differences were found in the
comparison of the latency to show grouping into a nest (KW(1) = 4.68, P
=
0.03; vehicle pretreatment latencies (median = 5.5 days) < naltrexone
pretreatment latencies (median = 10.0 days)).
Table 2.
Median sensitization latency to show full maternal behavior and
proportion of rats showing full maternal during the 10 day period of cohabitation
with pups in Expt. 1B.
|
Pretreatment
|
n
|
Median sensitization latency (in days)
|
Proportion of rats showing full maternal behavior within 10
days
|
|
Rats injected with 0.03 µg MS intra-VTA
|
| Naltrexone HCl (1 mg/kg, s.c.) |
6
|
10.0b
|
0.17
|
| Vehicle (1 ml/kg, s.c.) |
6
|
6.5
|
0.83
|
|
Rats injected with 0.03 µg MS dorsal to the VTA
|
| Vehiclea (1 ml/kg, s.c.) |
5
|
10.0b
|
0.20
|
aRats
received vehicle pretreatment for the purpose of comparison to the intra-VTA
MS-injected group receiving vehicle.
bSignificantly
different from vehicle pretreated rats receiving intra-VTA morphine (P
< 0.05). |
The vehicle-pretreated rats receiving
non-VTA morphine (5 dorsal, 1 anterior) showed long sensitization latencies
(median = 10 days) relative to those vehicle-pretreated rats receiving
VTA morphine (median = 6.5 days; KW(1) = 4.44, P = 0.035, dorsal
vs. VTA site). Therefore, the onset of full maternal behavior and of pup-grouping
were facilitated only in rats with cannula placements in the VTA.
The results of Exp. 1B strengthened
the conclusion drawn from Exp. 1A that an increase in opioid activity in
the VTA during the first few days of pup exposure hastens the onset of
maternal responsiveness. In Exps. 1A and 1B, rats treated with a single
injection of MS on each of the first three days of sensitization showed
full maternal behavior in little more than half the time of controls (5.5
to 6.5 days vs. > 10 days). Blockade of opioid receptors by pretreatment
with systemic naltrexone prevented the facilitating effect of MS on maternal
responsiveness, supporting an opioid-receptor specific site of action for
the MS. An alternative interpretation is that systemic naltrexone interfered
with the onset of maternal behavior through a mechanism independent of
the blockade of opioid receptors in the VTA. However, this interpretation
is not supported in previous studies by Mayer et al. [50]
which showed that systemic naltrexone and naloxone alone do not prevent
the onset of maternal behavior in the rat. Finally, the long sensitization
latencies in rats with cannula placements lying outside the VTA argue that
the site of the opioid-receptor field in which the intra-VTA morphine is
acting to facilitate sensitization latency is within the VTA.
5. Experiment 2
In Exp. 2, the effect of blocking
VTA opioid receptors on the onset of maternal behavior was tested during
the time when maternal behavior normally develops very rapidly -- the periparturitional
period. Forty min after parturition, mothers whose pups had been removed
at delivery were injected with the quaternary opioid antagonist naltrexone
methobromide (QN) bilaterally into the VTA. QN has been shown to diffuse
slowly from the site of injection in the CNS, and to be resistant to crossing
the blood-brain barrier [6,11,78].
Thirty min after the test rat was injected, foster stimulus pups were introduced
into the cage and the development of maternal behavior was observed.
5.1. Method
5.1.1. Subjects
Forty-seven postpartum primiparous
rats were divided into four groups: a QN-injected group; a vehicle-injected
group; a surgery-control group (sham treatment); and a no-treatment group.
5.1.2. Drugs
QN (MRZ 2663 BR, Batch H) was mixed
in 0.9% sterile saline. Bilateral injections consisted of 0.5 µg
in 0.5 m
l, injected at a rate of 1 m
l/min. The vehicle-injected rats received 0.5 m
l saline, injected at a rate of 1 m
l/min. This QN dose is within the range used previously to block other
opioid-mediated behaviors effectively when applied centrally [7,8,77].
5.1.3. Pretesting procedures
After the recovery period, accuracy
of intra-VTA cannula placement was assessed indirectly in 27 rats by determining
if a unilateral injection of MS (1 µg in 0.5 ml;
1 ml/min)
induced contralateral circling [26]
during a 1-h period after the injection. Two days after the first contralateral-circling
test, the rat was again tested, this time by injecting MS into the other
cannula. Any rat that failed to show a net increase in contralateral circling
after each MS injection was not tested further, since it was unlikely that
her cannulae placements fell in the VTA.
Approximately one week after the
two contralateral-circling tests, rats in proestrus were mated by co-housing
them with males overnight. Mating was considered successful if sperm was
found in the female's vaginal smear the next morning (Day 1 of pregnancy).
5.1.4. Testing procedures
On Day 21 of pregnancy, rats were
moved to delivery cages so they could habituate to them for at least 24
h. The characteristics of the delivery cage were identical to those of
the observation cages described above, except that the bottom was constructed
from a coarse wire mesh, so that pups would fall through the bottom of
the cage onto a soft mat (cotton batting) as they were delivered, thereby
allowing easy and unobtrusive removal by the experimenter. In addition,
a small, opaque, square of Plexiglas (10 X
10 X
0.3 cm) was placed in the cage to provide a platform on which the female
could easily adopt an anogenital-grooming/birthing posture. The pad was
not large enough to be occupied by both the female and the pups. Plexiglas
dividers were not used to partition the aquaria into quadrants as in Exps.
1A and 1B. Finally, great care was taken to remove the pups as soon as
possible, since it seemed in a prior test of this apparatus that the mother
became agitated by the presence of an out-of-reach pup on the floor beneath
the delivery cage. The parturient behavior of control rats in response
to these experimental procedures was similar to the parturient behavior
described by Dollinger et al. [12]
and by Kristal et al. [43] ;
for instance, delivery times fell within an expected range and females
remained relatively active during the immediate postpartum period.
Dams were observed continuously starting
on Day 22 of pregnancy for the appearance of labor and delivery. Forty
min postpartum, each dam received her assigned drug treatment and was returned
to her delivery cage, which was now equipped with a solid Plexiglas floor.
Bilateral injections were given consecutively using the same micro-infusion
pump used in Exps. 1A and 1B.
Pups were reintroduced to the female's
cage 30 min after the drug injection. To provide a stimulus similar to
that existing during parturition, pups were introduced with birth fluids
and tissues. The four foster pups were covered with a mixture of 1 ml amniotic
fluid and 4 placentas (minced and heated to 37 °
C) immediately before placing them in the test rat's cage for the first
time. Amniotic fluid and placenta from Day 21 pregnant females is regularly
collected and stored in our laboratory [42].
Fifteen-min behavior observations were conducted at 0, 2, 6, 12 h, and
subsequently every 12 h during the postpartum testing period. Pups were
replaced every 12 h with clean well-fed pups. During each behavior-observation
period, the presence or absence of full maternal behavior and pup grouping
was noted and the duration of pup-directed behavior, self-directed behavior,
other activity, and resting were determined as in Exp. 1A. In addition,
the duration of parturition, total number of pups, number of pups that
were cleaned off during parturition, and the number of placentas eaten
were recorded. The latter information was used to assure that the parturitional
experiences of rats in each group were similar. Group statistical comparisons
were made on latency data and on duration data obtained during the 0 and
2 h observation periods and also during the first observation period in
which full maternal behavior was observed.
5.2. Results and discussion
The contralateral-circling test indicated
that 18 of the 27 rats tested had bilateral cannulae terminating in the
VTA. In the 9 remaining rats, 8 showed contralateral circling after one
of the two circling tests, indicating that they were unilateral "hits"
only; one rat failed to show contralateral circling after either test,
indicating that both cannulae missed the VTA. Only rats shown to have bilateral
"hits" were used for the remainder of the experiment. The remaining 18
rats were combined with another 10 rats that had bilateral implants aimed
at the VTA but had not been screened in the contralateral-circling test,
and then randomly assigned to treatment conditions: 12 were assigned to
the experimental treatment group (QN), 8 to the vehicle-injection group,
and 8 to the sham-injection group. Because all control groups were expected
to perform similarly and because some rats were expected to be lost over
the course of pregnancy (see below), a larger number of rats was assigned
to the QN treatment group, so that at minimum, a statistical comparison
between rats in the experimental condition and rats in the combined control
conditions would be possible.
Thirty-eight rats were bred (18 contralateral
circlers, 10 non-screened VTA-implanted, and 10 no-treatment controls).
Of these, 24 carried their pregnancies to term and could be tested (the
remainder either failed to become pregnant, resorbed the fetuses, or sustained
damage to the cannula assembly; no trends relating treatment (surgery)
and the ability to initiate or maintain pregnancy were detected). Four
of the 24 were found upon histological examination to have unilateral or
bilateral cannula placement outside the VTA (none of the four had been
screened), leaving a total of 20 rats in which complete data were collected
and cannula placement was verified to lie within the VTA (Fig. 4). The
results obtained from these 20 rats (5 no-treatment rats, 6 QN-injection
rats, 4 no-injection rats, and 5 vehicle-injection rats) are reported here.
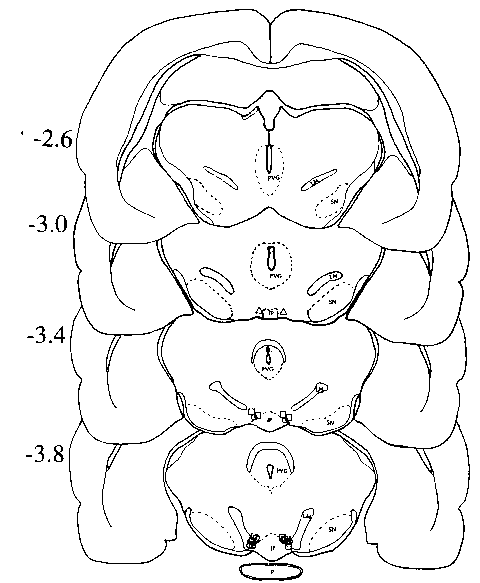
Fig 4. Schematic representation
of the location of cannula tips in Expt. 2. open square, QN treatment;
open circle, sham injection; open up triangle, no injection. Missed
placements not presented. IP, interpeduncular nucleus; LM, medial lemniscus;
MB, mammillary bodies; PVG, periventricular gray substance; P, pons; SN,
substantia nigra. Diagram after Pellegrino, et al. [56].
Because early pilot results indicated
that the extent to which the female was "distressed" during delivery would
affect her subsequent behavior toward the pups, several measures of delivery
were taken including day, time and duration of birth, total number of pups,
total number of pups with birth membranes removed ("clean pups"), and total
number of placentas eaten. The latter two measures also assessed indirectly
the amount of pup contact the female had during delivery. Regarding the
day and time of birth, 45% of the rats (9/20) gave birth on Day 22 of pregnancy,
55% (11/20) gave birth on Day 23, and all gave birth during the lights-on
phase of the light/dark cycle (as is usual). Table 3 presents the statistics
on the remaining birthing variables, by group.
Table 3.
Mean ± S.E.M. of several measures of labor and delivery in
Expt. 2.
Treatment
|
n
|
Duration of parturition (min) |
Proporrtion of whole placenta
eatena |
Proportion of total number of
pups cleanedb |
Litter size |
|
Naltrexone Methobromide
|
6
|
147.5 ± 12.7
|
0.63 ± 0.03
|
0.76 ± 0.06
|
10.5 ± 0.8
|
| Vehicle |
5
|
127.0 ± 27.2
|
0.86 ± 0.10
|
0.88 ± 0.09
|
7.6 ± 1.7
|
| Sham injection |
4
|
133.8 ± 38.6
|
0.80 ± 0.06
|
0.80 ± 0.08
|
10.0 ± 1.2
|
| No treatment |
5
|
158.0 ± 38.9
|
0.66 ± 0.10
|
0.71 ± 0.06
|
12.0 ± 0.5
|
| F3,16 |
< 1
|
2.05
|
1.10
|
2.61
|
| P |
|
0.15
|
0.38
|
0.09
|
a Number
of whole placenta eaten/number delivered.
b Number
of pups with birth membranes removed during delivery/number delivered. |
Overall, no significant group differences
in the birthing variables were found. Therefore, it seemed that the groups
had a similar parturition experience prior to any manipulation. In addition,
the periparturitional behavior of the 20 rats seemed comparable to the
periparturitional behavior of undisturbed and unmanipulated dams observed
in an another ongoing experiment in our laboratory, at least in terms of
the expected time of birth (during lights on), expected day of birth (evenly
split between Day 22 and Day 23 of pregnancy), and duration of birth (1
to 3 h with no periods of cessation greater than 30 min). Once the experimental
manipulation had been performed and foster pups introduced into the cages
of the postpartum females, maternal responsiveness was tested at 0, 2,
6, 12 h, and then every 12 h from the initial pup presentation until the
rats showed full maternal behavior. Maternal sensitization latency was
the primary dependent variable. Latency to show pup grouping was also recorded,
however it did not differ from latency to show full maternal behavior and
so was not analyzed statistically. In addition, as in Exps. 1A and 1B,
the time spent in various behaviors during each observation period was
measured.
5.2.1. Maternal responsiveness
The median sensitization latencies
and cumulative percent of rats showing full maternal behavior at each test
are presented in Fig. 5. QN-treated rats took significantly longer to show
maternal behavior than did controls (QN: median = 30 h; Vehicle: median
= 2 h; No Injection: median = 4 h; No Treatment: median = 6 h) (KW(3) =
14.3, P = 0.0025). As predicted, blockade of opioid receptors in
the VTA during the natural onset of maternal behavior significantly delayed
the onset of maternal responsiveness.
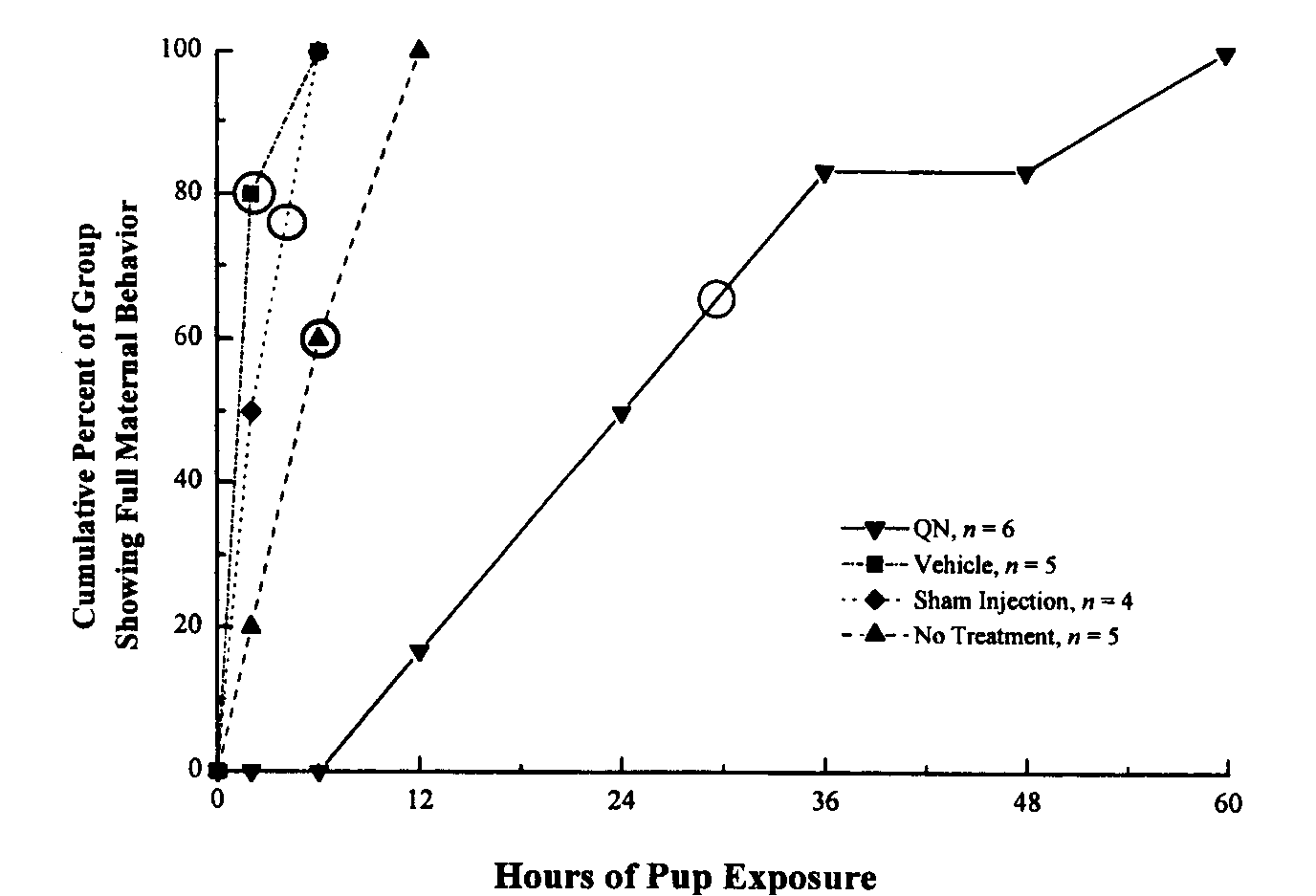
Fig. 5. Cumulative percent of group showing full maternal
behavior after 0, 2, 6, 12, 24, 36, 48, and 60 h of pup exposure. Rats
were given bilateral intra-VTA injections of either naltrexone methobromide
(QN), vehicle, sham injection, or no treatment, at 40 min postpartum (30
min before pup presentation). The dams' own pups had been removed during
parturition to slow or prevent the development of maternal behavior. No
rat showed full maternal behavior during the first postpartum test. The
median latency of each group is indicated by an open circle. The QN group
showed a significantly slower onset of full maternal behavior than did
the control groups (P < 0.05). Control groups did not differ
significantly from each other (P > 0.05).
5.2.2. Other behavioral measures
The data from the 0 h observation
period, the 2 h observation period, and the observation period in which
full maternal behavior was observed were chosen for analysis, and are presented
in Fig. 6. During the first observation (0 h), 30 min after the drug infusion
and immediately after the first re-exposure to rat pups, no differences
in either pup-directed behavior or in total active behavior were found
(pup-directed behavior: F3,16 <
1; total active behavior: F3,16 =
1.47, P = 0.25).
At the second observation (2 h),
2.5 h after drug injection, a trend toward lower total active behavior
was observed in QN-treated rats, however, the number of rats not yet showing
full maternal behavior in control groups at this time was too small to
assess statistically. Instead, data from the control groups were combined
and then compared to the QN group. In these analyses, no differences in
pup-directed behavior were found between untreated (37.6 ± 29.3
s) and control-treated (121.3 ± 80 s) rats (F1,11
<
1], and a significant decrease in total active behavior was found between
QN-injected (212.3 ± 106.1 s) and control-treated (707.8 ±
102.8 s) rats (F1,11=
11.16, P = 0.006).
No group differences in the duration
of the ancillary behaviors were apparent during the observation period
in which full maternal behavior was first observed (pup-directed behavior:
F3,16
=
2.52,
P = 0.09; total active behavior: F3,16
< 1).
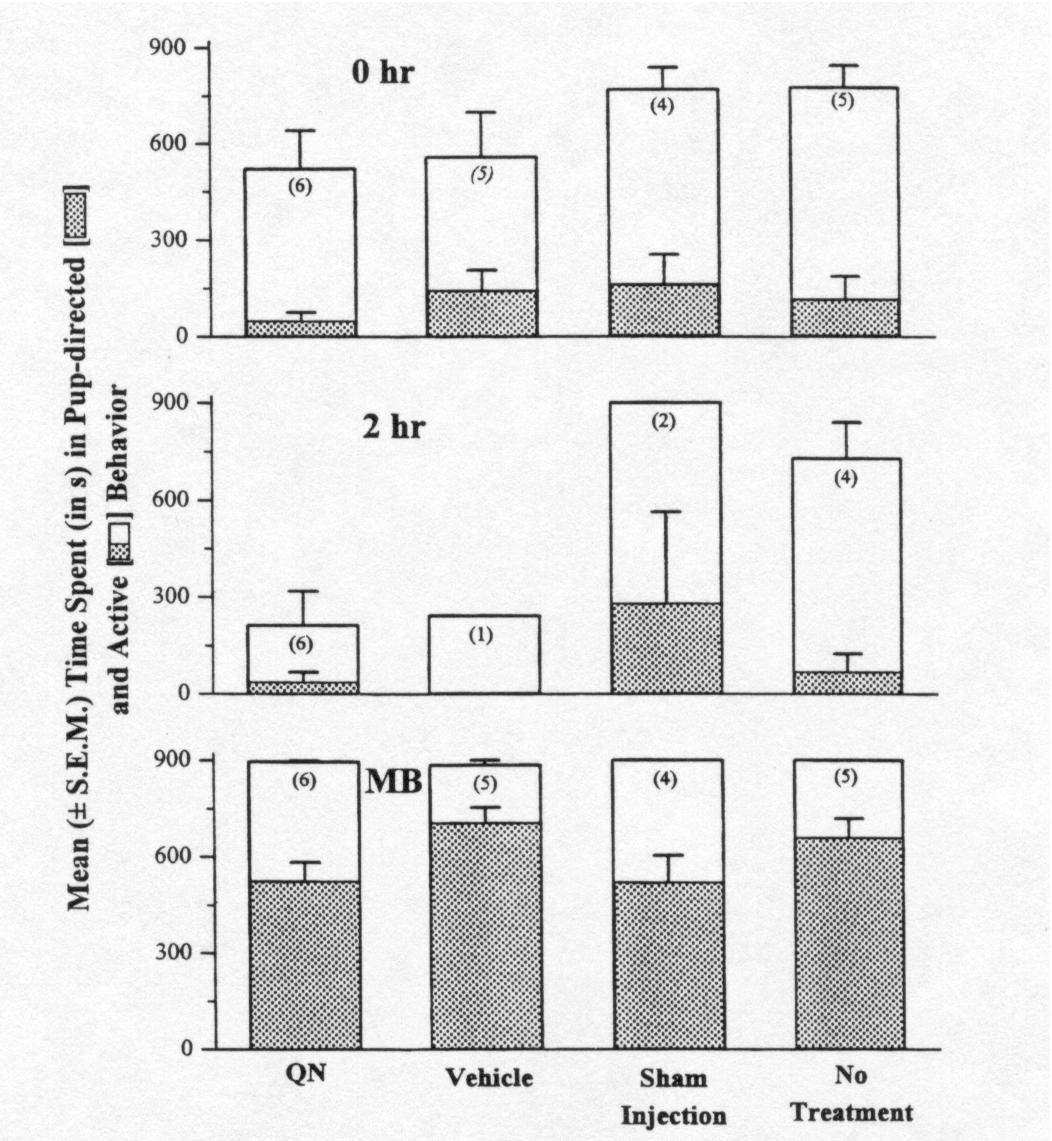
Fig. 6 . Mean ± S.E.M. time spent (in s) in pup-directed
or active behavior during the 15-min tests at 0 h and 2 h after pup presentation,
and during the test in which full maternal behavior was observed (MB).
Rats were treated with either naltrexone methobromide (QN), vehicle, sham
injection, or no treatment at 40 min postpartum (30 min before pup presentation).
The number in parentheses represents the number of rats observed on that
test (rats showing maternal behavior before the 2-h test were not among
those tested at 2 h). No significant differences among groups in either
pup-directed behavior or active behavior were found at 0 h or MB. At 2
h, the QN-treated rats showed significantly less active behavior than did
the combined controls (P < 0.006) but did not differ in pup-directed
behavior.
These results strongly support the hypothesis
that increased opioid activity in the VTA is necessary for the almost immediate
onset of maternal behavior during the periparturitional period, as blockade
of opioid activity in the VTA at that time impedes the natural onset of
maternal responsiveness. No significant differences between treatment groups
were found in the duration of various ancillary behaviors observed during
the first observation (0 h) and, therefore, there are no obvious changes
in behavior that are correlated with the difference in maternal responsiveness.
At this time, all rats were observed to approach, sniff, and clean the
placenta-covered pups, and no rat showed marked lethargy or aberrant stereotypic
behavior. The significant decrease in total active behavior observed during
the 2-h observation period was not correlated with sensitization latency;
control-treated rats showing the least total active behavior (similar to
the QN-injected rats) were nearest to showing full maternal behavior. In
this observation period, any rat, regardless of drug treatment, not engaged
in some pup-directed behavior was observed to be resting in a typical sleep
posture. Overall, this data argues against the possibility that a decrease
in pup contact (passive or active) delayed the sensitization process in
QN-injected rats.
The delay in the onset of maternal
behavior among QN-treated rats was longer than expected given previous
reports on the time course of QN drug action. Peak effects of QN on other
opioid-mediated behaviors have been obtained within 15 to 30 min of drug
administration [7,8,44,77]. We
reintroduced pups 30 min after the QN or control injection, and found that
more than 90 % of the control dams exhibited full maternal behavior within
6 h, whereas the fastest QN-treated dam showed full maternal behavior only
after 12 h. If the time course for action of QN is similar to that reported
for other opioid-mediated responses, then the appearance of maternal behavior
among QN-treated rats required considerably more time than can be explained
by the acute action of QN. This suggests that the effect of QN treatment
was on the development of maternal behavior and not simply its expression.
Although maternal behavior appears rapidly during parturition because of
the facilitation effect of the parturitional, hormonal, and neurochemical
milieu, a number of studies have demonstrated that in the absence of this
facilitating milieu, as in the virgin rat, the induction of maternal behavior
involves a gradual development of sensitivity toward pup stimuli [52,68].
In the natural context, this begins during parturition and the immediate
postpartum period when the dam has extensive contact with the pups in the
processes of delivery and of cleaning and ingesting afterbirth materials.
The importance of this contact is apparent when pup contact is reduced
during parturition (as in this experiment) and the capacity of dams to
respond to pup stimuli at a later time is hampered. By reducing pup contact
during parturition, the appearance of full maternal behavior required several
hours of subsequent exposure to pups. Recently, Fleming and Sarker [17]
have shown that the first 2 h of postpartum pup exposure are critical for
subsequent rapid induction of full maternal behavior. Disruption of mother-pup
contact during this period increases significantly the sensitization latencies
to pups at a later time. Perhaps a similar phenomenon occurred in this
experiment. The advantage conferred to dams by pup contact during the immediate
postpartum period was blocked by opioid reduction due to QN treatment,
and so a longer period of development was required before full maternal
behavior was exhibited in this group.
Observations made during the initial
15 min of postpartum pup exposure in Exp. 2 revealed no significant differences
in the amount time spent with pups, and all dams were observed to lick
and ingest the afterbirth materials. Casual notes taken during this period,
however, suggested that QN-treated dams were less responsive to pup stimuli.
These dams did not avoid stepping on pups, and showed no response to the
pups when they did step on them. In contrast, dams in other treatment groups
typically "walked around" pups and "jumped back" if they did stepped on
one. However, once full maternal behavior was expressed, no differences
in the quantity and quality of maternal behavior were found. In summary,
QN treatment interfered with the development of maternal behavior during
the immediately postpartum period, but did not produce a long-term change
in the rat's capacity to develop this behavior or the quality and quantity
of maternal behavior once it was established.
6. General Discussion
The results of Exps. 1A and 1B show
that increased opioid activity in the VTA is sufficient to reduce the amount
of pup exposure needed to induce maternal behavior in virgin rats. Among
rats treated with the most effective dose of morphine, the median sensitization
latency was half that of control rats receiving no treatment, vehicle treatment,
or an ineffective (low) dose of morphine. No evidence was obtained to suggest
that the facilitating effect of morphine on maternal responsiveness was
limited to the acute actions of the drug: no rat showed maternal behavior
within the drug-injection period and grouping behavior was not dissociated
from the onset of maternal responsiveness among drug-treated rats. Nor
was any evidence obtained to suggest that the effect of morphine on maternal
responsiveness was the result of the formation of a conditioned association
between the presence of pups and the application of morphine: no group
differences were obtained in the amount of time spent with pups, and no
change in the females' responsiveness to novel objects, other than pups
that were paired with the drug application, were observed at any time.
Finally, no significant differences in activity level were observed among
groups after three days of drug treatment, so incidental increases in pup
contact due to an increase in general activity seem unlikely. Therefore,
increased opioid activity in the VTA facilitated the onset of maternal
responsiveness in virgin rats without overtly increasing pup contact.
The facilitating effect of intra-VTA
MS on maternal responsiveness was shown to be specific to VTA and not the
result of activation in areas immediately dorsal, anterior, or lateral
to the injection site (Exp. 1A and 1B). Furthermore, the facilitating effect
of intra-VTA MS appeared to be opioid-receptor mediated as the effect was
blocked by pretreatment with systemically administered naltrexone.
In Exp. 2, blockade of opioid receptors
in the VTA blocked maternal responsiveness during the periparturitional
period without changing the amount of initial total activity or pup contact.
This strongly suggests that opioid activity in the VTA is necessary for
the rapid onset of maternal behavior associated with the periparturitional
period.
Together these results provide evidence
for a stimulatory role for endogenous opioids in the development of maternal
behavior. This would be in agreement with several papers on the initiation
of maternal behavior in the sheep [35,37] and
the expression of some maternal behaviors in the primate [48,63]
and
the rat [43,50]. These research
findings seem to be in contrast with those of Bridges who showed that morphine
produced an inhibitory effect on maternal behavior through its action on
m-opioid
receptors in the medial preoptic area [5,20,45,46,62].
These seemingly conflicting results may reflect the difference in neuromechanism
mediating the development and expression of maternal behavior. Another
possibility is that facilitating or inhibiting effect of opioids are mediated
by different neural substrates under different physiological conditions
[66].
The dissociation between drug treatment
and the eventual appearance of maternal behavior suggests that the results
obtained in these experiments do not reflect an acute drug effect on the
expression of maternal behavior. No virgin rat displayed full maternal
behavior or any component of maternal behavior during the several-hour
period following morphine microinjection into the VTA. Furthermore, in
postpartum dams the appearance of maternal behavior after blockade of VTA
opioid receptors occurred many hours later. Given the short action of the
antagonist treatment, it seems that blockade of VTA opioid receptors did
not simply prevent the expression of maternal behavior; instead it apparently
prevented the natural development of maternal behavior.
It is well established that microinjection
of opioid agonists or antagonists into the VTA change A10 dopamine neurochemistry,
increasing or decreasing dopamine levels respectively in the n. accumbens
[9,10,21,33,49]. Furthermore,
repeated opioid stimulation produces long term changes in mesolimbic dopaminergic
activity [34]. Therefore, one
possible mechanism of action for intra-VTA opioid stimulation or inhibition
would be to increase and decrease dopamine neurotransmission.
Manipulations that increase or decrease
mesolimbic dopamine activity affect a wide variety of motivated behaviors,
including maternal behavior [3,24,34,51].
Disruption of the DA terminals in the n. accumbens by electrolytic lesion
[65] or by 6-OHDA lesion
[22-25] decreases the expression and
initiation of maternal behavior. Dopamine receptor blockade by haloperidol
disrupts some aspects of maternal behavior[19],
whereas stimulation of dopamine, indirectly by tail pinch [1],
facilitates the development of maternal behavior [71].
In contrast, cocaine, which dramatically increases dopamine in the n. accumbens
and elsewhere, does not facilitate the onset or expression of maternal
behavior and, in fact, has a negative effect on the development and expression
of maternal behavior [32,39].
In these latter studies, however, cocaine was administered systemically,
so it may have produced competitive effects at different neuroanatomical
or neurochemical sites. Overall, the available data on dopaminergic manipulations
within the mesolimbic system support the idea that the development and
expression of maternal behavior are mediated in part by this dopaminergic
system. Our data would lend some support in that it is well established
that endogenous opioids regulate dopaminergic neurotransmission at the
level of the VTA.
Manipulation of the mesolimbic dopmaine
system affect a broad range of behaviors; consequently, several theories
have been developed to provide a unifying conceptual framework in which
to view the role of the mesolimbic dopamine system in behavior, all of
which emphasize the importance of this substrate in mediating sensory-motor
responses
[3,34,51,81]. A fundamental characteristic
of maternal behavior is that it is stimulus-induced and stimulus-dependent
[60,61,82]. Considerable research has
suggested that among nullipara females, olfactory stimuli inhibit the onset
of maternal responsivness [16],
whereas somatosensory stimuli facilitate the onset of maternal responsiveness
[36,69]. Interestingly, West and Michael
[81] have shown that increases
in n. accumbens dopamine selectively modify the electrophysiologocal response
to somatosensory and olfactory stimuli. Perhaps in the experiments presented
here, opioid manipulation modified the sensory quality of pup contact.
Therefore, although total pup contact did not change, differences in the
effect of pup contact may have existed. In Exps. 1A and 1B, intra-VTA morphine
may have increased mesolimbic dopaminergic activity and enhanced the tactile
sensory stimuli emitted by the pups, thereby facilitating the development
of maternal behavior. In Exp. 2, blockade of VTA opioid receptors may have
disturbed the initiation of maternal behavior by blocking the stimulating
actions of endogenous opioids on mesolimbic dopamine, which in turn may
have reduced the effects of pup contact.
No evidence is available to show
whether endogenous opioid levels in the VTA are changed during the immediate
postpartum period. However, endogenous opioid activity is greatly affected
by gonadal steriods [58,80].
Wardlaw and Frantz [80] suggested
that dynamic changes in midbrain opioid activity do occur around parturition,
reporting that increases in opioid levels in the midbrain occur during
later pregnancy, peak near parturition, and decrease in the first 24-48
hr postpartum. Additional research will be necessary to determine if similar
changes occur in the VTA and if so, whether these changes reflect an increase
in opioid release that is correlated with the period when maternal behavior
develops.
In conclusion, the data presented here provide further support for the
importance of the VTA in the underlying neuroanatomic substrate mediating
maternal behavior. Furthermore, these results add to a growing body of
evidence that suggest that endogenous opioids participate in significant
ways in periparturitional behavior, in this case by facilitating the onset
of maternal behavior through action in the VTA.
Acknowledgments
This research was supported by NSF Grants BNS 88-19837 and IBN 91-23748
awarded to M.B.K., and a Mark Diamond Fund Award to A.C.T. The authors
thank Michael Bozarth for his helpful advice; Herbert Merz and Boehringer
Ingelheim, A.G., for generously donating quaternary naltrexone; and Jean
DiPirro and Patricia Abbott for their assistance. The protocols used herein
were approved by the IACUC of the State University of New York at Buffalo.
Footnote
* Corresponding author. Present address: Department of Psychology, University
at Buffalo, Buffalo, NY 14260, USA. E-mail: ACT2@acsu.buffalo.edu.
References
-
Boutelle, M.G., Zetterstrom T., Pei, Q., Svensson, L., and Fillenz, M.,
In vivo neurochemical effects of tail pinch. J. Neurosci. Meth., 34
(1990) 151-157.
-
Bozarth, M.A., Neuroanatomical boundaries of the reward-relevant opiate
receptor field in the ventral tegmental area as mapped by conditioned place
preference. Brain Res., 414 (1987) 77-84.
-
Bozarth, M.A., The mesolimbic dopamine system as a model reward system.
In P. Willner and J. Scheel-Kruger (Eds.), The mesolimbic dopamine system:
From motivation to action, John Wiley and Sons, New York, 1991, 299-328.
-
Bridges, R.S., Parturition: Its role in the long term retention of maternal
behavior in the rat, Physiol. Behav., 18 (1977) 487-490.
-
Bridges, R.S. and Grimm, C.T., Reversal of morphine disruption of maternal
behavior by concurrent treatment with the opiate antagonist naloxone, Science,
218
(1982)
166-168.
-
Brown, D.R. and Goldberg, L.I. The use of quaternary narcotic antagonists
in opiate research, Neuropharmacology, 24 (1985) 181-191.
-
Brown, D.R. and Holtzman, S.G., Opiate antagonists:central sites of action
in suppressing water intake of the rat, Brain Res., 221 (1981) 432-436.
-
Brown, D.R., Robertson, M.J., and Goldberg L.I., Reversal of morphine-induced
catalepsy in the rat by narcotic antagonists and their quaternary derivatives,
Neuropharmacology,
22 (1983) 317-321.
-
Cador, M., Rivet, J-M., Kelly, A.E., Le Moal, M. and Stinus, L., Substance
P, neurotensin and enkephalin injections into the ventral tegmental area:
Comparative study on dopamine turnover in several forebrain structures,
Brain
Res., 486 (1989) 357-363.
-
Dilts, R.P. and Kalivas, P.W., Autoradiographic localization of ?-opioid
and neurotensin receptors within the mesolimbic dopamine system, Brain
Res., 488 (1989) 311-327.
-
DiPirro, J.M., Thompson, A.C. and Kristal, M.B. Amniotic fluid (POEF) ingestion
enhances morphine analgesia centrally but not peripherally, Brain Res.
Bull., 26 (1991) 851-855.
-
Dollinger, M.J., Holloway, W.R. Jr. and Denenberg, V.H., Parturition in
the rat (Rattus norvegicus): Normative aspects and the temporal
patterning of behaviors, Behav. Proc., 5 (1980) 21-37.
-
Fahrbach S.E. and Pfaff, D.W., Hormonal and neural mechanisms underlying
maternal behavior in the rat. In D.W. Pfaff (Ed.), The physiological
mechanism of motivation, Springer-Verlag, New York, 1982, pp. 253-285.
-
Fleiss, J.L. Statistical methods for rates and proportions. Wiley,
New York, 1981.
-
Fleming, A.S. and Rosenblatt, J.S., Maternal behavior in the virgin and
lactating rat, J. Comp.Physiol. Psychol., 86 (1974) 957-972.
-
Fleming A.S. and Rosenblatt J.S., Olfactory regulaton of maternal behavior
in rats: effects of peripherally induced anosmia and lesions of the lateral
olfactory tract in pup-induced virgins, J. Comp. Physiol. Psych, 86
(1974)
233-246.
-
Fleming, A.S., and Sarker, J., Experience-hormone interactions and maternal
behavior in rats, Physiol. Behav., 47 (1990) 1165-1173.
-
Gaffori, O., and Le Moal, M., Disruption of maternal behavior and appearance
of cannibalism after ventral mesencephalic tegmentum lesions. Physiol.
Behav., 23 (1979) 317-323.
-
Giordano, A.L., Johnson, A.E., and Rosenblatt, J.S., Haliperidol-induced
disruption of retrieval behavior and reversal with apomorphine in lactational
rats, Physiol. Behav., 48 (1990) 211-214.
-
Grimm, C.T. and Bridges, R.S., Opiate regulation of maternal behavior in
the rat, Pharmacol. Biochem. Behav., 19 (1983) 609-616.
-
Gysling, K. and Wang, R.Y., Morphine-induced activation of A10 dopamine
neurons in the rat, Brain Res., 277 (1983) 119-127.
-
Hansen, S., Maternal behavior of female rats with 6-OHDA lesions in the
ventral striatum: characterization of the pup retrieval deficit, Physiol.
Behav., 55 (1991) 6125-620
-
Hansen, S., Bergvall, Å.H. and Nyiredi, S. Interaction with pups
enhances dopamine release in the ventral striatum of maternal rats: A microdialysis
study. Pharmacol. Biochem. Behav., 45 (1993).
-
Hansen, S., Harthon, C., Wallin, E., Loftberg,L. and Svensson, K., The
effect of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum
on maternal and sexual behavior in the female rat. Pharmacol. Biochem.
Behav. 39 (1991) 71-77.
-
Hansen, S., Harthon, C., Wallin, E., Loftberg, L. and Svensson, K., Mesotelencephalic
dopamine system and reproductive behavior in the female rat: Effects of
ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness,
Behav.
Neurosci., 105 (1991) 588-598.
-
Holmes, L.J. and Wise, R.A., Contralateral circling induced by tegmental
morphine: Anatomical localization, pharmacological specificity, and phenomenology,
Brain
Res., 326 (1985) 19-26.
-
Jakubowski, M. and Terkel, J., Pup and object carrying by maternally and
nonmaternally behaving female albino rats (Rattus norvegicus),
J. Comp. Psych., 98 (1984) 331-317.
-
Jakubowski, M. and Terkel, J., Incidence of pup killing and parental behavior
in virgin female and male rats (Rattus norvegicus): Differences between
Wistar and Sprague-Dawley stocks. J. Comp. Psych., 99 (1985) 93-97.
-
Jenck, F., Bozarth, M., and Wise, R.A., Contraversive circling induced
by ventral tegmental microinjections of moderate doses of morphine and
[D-Pen2,D-Pen5]enkephalin, Brain Res., 450
(1988) 382-386.
-
Jenck, F., Gratton, M. A. and Wise, R.A., Opposite effects of ventral tegmental
and periaqueductal gray morphine injections on lateral hypothalamic stimulation-induced
feeding, Brain Res., 399 (1986) 24-32.
-
Jenck, F., Gratton, A. and Wise, R.A., Opioid receptor subtypes associated
with ventral tegmental facilitation and periaqueductal gray inhibition
of feeding, Brain Res., 423 (1987) 39-44.
-
Johns, J.M., Noonan, L.R., Zimmerman, L.I., Li L., and Pedersen, C.A.,
Effects of chronic and acute cocaine treatment on the onset of maternal
behavior and aggression in Sprague-Dawley rats, Behav. Neurosci. 108
(1994) 107-112.
-
Kalivas, P.W., Interactions between neuropeptides and dopamine neurons
in the ventromedial mesencephalon, Neurosci. Biobehav. Rev., 9,
(1985) 573-578.
-
Kalivas, P.W. and Stewart, J., Dopamine transmission in the initiation
and expression of drug- and stress-induced sensitization of motor activity.
Brain
Res. Rev., 16 (1991) 223-244.
-
Kendrick, M.K. and Keverne, E.B., Effects of intracerebroventricular infusions
of naltrexone and phentolamine on central and peripheral oxytocin release
and on maternal behaviour induced by vaginocervical stimulation in the
ewe, Brain Res., 505 (1989) 329-332.
-
Keynon, P. Cronin P., and Keebles S., Disruption of maternal retrieving
by perioaral anesthesia, Physiol. Behav., 27, (1981) 313-321.
-
Keverne, E.B. and Kendrick, M.K., Morphine and corticotrophin-releasing
factor potentiate maternal acceptance in multiparous ewes after vaginocervical
stimulation, Brain Res., 540 (1991) 55-62.
-
Kimball, C.D., Do endorphin residues of beta-lipotropin in hormone reinforce
reproductive function?, Am. J. Obstet. Gynecol., 143 (1979) 127-132.
-
Kinsley, C.H., Turco, D., Bauer, A., Beverly, M, Wellman, J., and Graham,
A.L., Cocaine alters the onset and maintenance of maternal behavior in
lactating rats, Pharm. Biochem. Behav., 47 (1994) 857-864.
-
Kinsley, C.H., Wellman, J.C., Carr, D.B. and Graham, A., Opioid regulation
of parental behavior in juvenile rats, Pharmacol. Biochem. Behav.,
44
(1993) 763-768.
-
Kristal, M.B., Enhancement of opioid-mediated analgesia: A solution to
the enigma of placentophagia, Neurosci. Biobehav. Rev., 15 (1991)
425-435.
-
Kristal, M.B., Abbott, P. and Thompson, A.C., Dose-dependent enhancement
of morphine-induced analgesia by ingestion of amniotic fluid and placenta,
Pharmacol.
Biochem. Behav., 31 (1988) 351-356.
-
Kristal, M.B., Thompson, A.C., Abbott, P., DiPirro, J. M., Ferguson, E.J.
and Doerr, J.D., Amniotic-fluid ingestion by parturient rats enhances pregnancy-mediated
analgesia, Life Sci., 46 (1990) 693-698.
-
Locke, K.W. and Holtzman, S.G., Characterization of the discrimative stimulus
effects of centrally administered morphine in the rat. Psychopharmacology,
87 (1985) 1-6.
-
Mann, P.E., Kinsley, C.H. and Bridges, R.A., Opioid receptor subtype involvement
in maternal behavior in lactating rats. Neuroendocrinology,
53
(1991) 487-492.
-
Mann, P.E., Pasternak, G.W. and Bridges, R.S., Mu opioid receptor involvement
in maternal behavior in lactating rats. Physiol. Behav., 47 (1990)
133-138.
-
Mansour, A., Fox, C.A., Akil, H., and Watson, S.J., Opioid-receptor mRNA
expression in the rat CNS: anatomical and function implications, Trends
Neurosci., 18 (1995) 22-29.
-
Martel, F.L., Nevison, C.M., Rayment, F.D., Simpson, M.J.A. and Keverne,
E.B., Opioid receptor blockade reduces maternal affect and social grooming
in rhesus monkeys, Psychoneuroendocrinology, 18 (1993) 307-321.
-
Matthews, R.T. and German, D.C., Electrophysiological evidence for excitation
of rat ventral tegmental area dopamine neurons by morphine, Neuroscience,
11
(1984) 617-625.
-
Mayer, A.D., Faris, P.L., Komisaruk, B.R. and Rosenblatt, J.S., Opiate
antagonism reduces placentophagia and pup cleaning by parturient rats,
Pharmacol.
Biochem. Behav., 22 (1985) 1035-1044.
-
Mogenson, G.J., Jones, D.L. and Yim, C.Y., From motivation to action: functional
interface between the limbic system and the motor system, Progress in
Neurobiology, 14 (1980) 69-97.
-
Numan, M., Maternal behavior. In E. Knobil, and J. Neill, et al. (Eds.),
The
physiology of reproduction, (2nd ed.), Raven Press, New York, 1994,
pp. 221-302.
-
Numan, M. and Corodimas, K.P., The effects of paraventricular hypothalamic
lesions on maternal behavior in rats, Physiol. Behav., 35 (1985)
417-425.
-
Numan, M. and Smith, H.G., Maternal behavior in rats: Evidence for the
involvement of preoptic projections to the ventral tegmental area, Behav.
Neurosci., 98 (1984) 712-727.
-
Orpen, B.G. and Fleming A.S., Experience with pups sustains maternal responding
in postpartum rats. Physiol. Behav., 40 (1987) 47-54.
-
Pellegrino, L.J., Pellegrino, A.S. and Cushman, A.J., A stereotaxic
atlas of the rat brain, Plenum Press, New York, 1979.
-
Peters, L.C. and Kristal, M.B., Suppression of infanticide in mother rats,
J.
Comp. Psychol., 97 (1983) 167-177.
-
Petraglia, F., Baraldi, M., Giarre, G., Facchinetti, F., Santi, M., Volpe,
A. and Genazzani, A.R., Opioid peptides of the pituitary and hypothalamus:
Changes in pregnant and lactating rats, J.Endo.,105 (1985) 239-245.
-
Phillips, A.G. and LePiane, F.G., Reinforcing effects of morphine microinjection
into the ventral tegmental area, Pharmacol. Biochem. Behav., 12
(1980) 965-968.
-
Rosenblatt, J.S., Nonhormonal basis of maternal behavior in the rat,
Science,
156 (1967) 1512-1514.
-
Rosenblatt, J.S. and Lehrman, D.S., Maternal behavior of the laboratory
rat. In H.L. Rheingold (Ed.), Maternal behavior in mammals, John
Wiley and Sons, New York, 1963, pp. 8-57.
-
Rubin, B.S. and Bridges, R.S., Disruption of ongoing maternal responsiveness
in rats by central administration of morphine sulfate, Brain Res.,
307
(1984) 91-97.
-
Schino, G. and Troisi, A., Opiate receptor blockade in juvenile macaques:
Effect on affiliative interactions with their mothers and group companions,
Brain
Res., 576 (1992) 125-130.
-
Siegel, S. and Castellan, N.J., Jr., Nonparametric statistics for the
behavioral sciences, (2nd ed.). McGraw-Hill, New York, 1978.
-
Smith M.O. and Holland R.C., Effects of lesions of the nucleus accumbens
on lactation and postpartum behavior, Physiol. Psych., 3 (1985)
331-336.
-
Spanagle R. Herz A., and Shippenberg, T.S., Opposing tonically active endogenous
opioid systems modulate the mesolimbic dopamine pathway, Proc. Natl.
Acad. Sci. USA, 89 (1992) 2046-2055.
-
Stern, J.M., Maternal behavior priming in virgin and ceasarean-delivered
Long-Evans rats: Effects of brief contact or continuous exteroceptive pup
stimulation, Physiol. Behav., 31 (1983) 757-764.
-
Stern, J.M., Maternal behavior: Sensory, hormonal, and neural determinants.
In S. Levine and F.R. Brush (Eds.), Psychoendocrinology, Academic
Press, New York, 1988, pp. 1- 104.
-
Stern, J.M., and Kolunie, J.M., Perioral anesthesia disrupts maternal behavior
during early lactation. Behav. Neural Biol., 52 (1989) 20-38.
-
Steuer, M.A., Thompson, A.C., Doerr, J.C., Youakim, M. and Kristal, M.B.,
Induction of maternal behavior in rats: Effects of pseudopregnancy termination
and placenta-smeared pups, Behav. Neurosci., 101 (1987) 219-227.
-
Szechtman, H. Siegel, H.I., Rosenblatt, J.S., and Komisaruk, B.R., Tail-pinch
facilitates onset of maternal behavior in rats, Physiol. Behav.,
19
(1977) 807-809.
-
Tabachnick, B.G. and Fidell, L.S., Using multivariate statistics,
(2nd ed.), Harper & Row, Cambridge, 1989.
-
Tarapacki, J.A. and Kristal, M.B., An adaptable microcomputer program for
recording behavior durations, Physiol. Behav., 47 (1990) 381-384.
-
Tarapacki, J.A. and Kristal, M.B., An adaptable microcomputer program for
monitoring behavior, Physiol. Behav., 53 (1993) 621-622.
-
Terkel, J. and Rosenblatt, J.S., Aspects of non-hormonal maternal behavior
in the rat. Horm. Behav., 2 (1971) 161-171.
-
Thompson, A.C., and Kristal, M.B., Opioids in the ventral tegmental area
facilitate the onset of maternal behavior in the rat. Society for Neuroscience
Abstracts, 18, Part 1, (1992) 539.
-
Ukai, M. and Holtzman, S.G., Suppression of deprivationn-induced water
intake in the rat by opioid antagonists: central sites of action, Psychopharmacology,
91 (1987) 279-284.
-
Valentino, R.J., Herling, S., Woods, J.H., Medzihradsky, F. and Merz, H.
Quaternary naltrexone:Evidence for the central mediation of discriminative
stimulus effects of narcotic agonists and antagonists, J. Pharmacol.
Exp. Ther. 217 (1981) 652-659.
-
Vezina, P. and Stewart, J., Conditioning and place-specific sensitization
of increases in activity induced by morphine in VTA, Pharm. Biochem.
Behav,. 20 (1984) 925-934.
-
Wardlaw, S.L. and Frantz, A.G., Brain b-endorphin
during pregnancy, parturition, and the postpartum period, Endocrinology,
113 (1983) 1664-1668.
-
West, C.H.K. and Michael, R.P., Responses of units in the mesolimbic system
to olfactory and somatosensory stimuli: modulation of sensory input by
ventral tegmental stimulation, Brain Res., 532 (1990) 307-316.
-
Wiesner, B. P. and Sheard, N.M., Maternal behavior in the rat. Oliver
and Boyd, Edinburgh, 1933.





