FIG. 1. Mean Shocks and Mean Responses per 5 min block per reproductive condition, across phases.
Learning in Escape/Avoidance Tasks in
Female Rats Does Not Vary With
Reproductive Condition1
MARK B. KRISTAL,*2 SEYMOUR AXELROD† AND MICHAEL NOONAN*
*Department of Psychology and †Department
of Psychiatry
State University of New York at Buffalo, Buffalo,
NY 14226
(Received 1 February 1977)
KRISTAL, M. B., S. AXELROD AND M. NOONAN. Learning in escape/avoidance tasks in female rats does not vary with reproductive condition. PHYSIOL. BEHAV. 21(2) 251-256, 1978. -- To determine whether the development of novel stimulus-response associations by the mother during the periparturient period is attributable to a general facilitation of learning produced by the hormonal milieu during that period, learning ability under various reproductive conditions was assessed in two tasks unrelated to the periparturitional situation. The two tasks, selected because they equalized the various groups for motivation and performance variables, were acquisition of a water-maze escape (including two reversals), and acquisition and retention of an unsignalled shuttlebox shock avoidance. The groups tested in the water maze were a midpregnant group, an immediately prepartum group, and an immediately postpartum group. In the shuttlebox, the same conditions (different rats) were compared, together with a nonpregnant estrus condition, and a nonpregnant diestrus condition. The results of both experiments indicate that although learning occurred, the characteristics of acquisition and retention were not influenced by reproductive condition.Pregnancy Avoidance Escape Shuttlebox Diestrus Water maze
Maternal behavior Estrus Estrous cycle Learning
PERINATAL maternal behavior in the rat, as in other altricial mammals, is characterized by an elaborate constellation of caretaking behaviors including placentophagia, retrieval of the neonates to a central nest site, licking of the neonates (especially in the anogenital area), hovering or crouching over the neonates to allow them to nurse, and inhibiting foot and jaw movements that otherwise would lead to injury to the offspring [28,35]. Although the specific motor responses performed by the mother are not novel, the performance of these motor acts in response to the novel stimuli that exist at the first parturition (placenta, pups, etc.) represents a series of entirely new stimulus-response associations. These new associations arise rapidly in the parturient rat, being evident within minutes of the delivery of the first neonate [28]. These associations are not evident, however, in parturitionally inexperienced females that are not at least on the verge of giving birth. Virgin female rats require days of constant exposure to rat pups before the maternal behaviors are manifested [27]. The difference in latency to maternal behavior between the inexperienced parturient rat and the inexperienced nonpregnant rat has been assumed to be due primarily to the hormonal milieu of the parturient rat; and it has been shown that making the hormonal milieu of the virgin more like that of the parturient female shortens the latency [16,22,30,31,32,36].
The effect of previous parturitional experience on the permanence of maternal behavior is profound. Once the maternal behaviors have been practiced, particularly if they were initiated during the perinatal period, they are subsequently readily elicitable by the external stimuli, even in the absence of brain structures, glands, or hormones that were necessary for the original appearance of the behaviors [4,5,11,15,18,19,23,29].
The development of the novel associations between old motor patterns and new stimuli, and the subsequent incorporation of these stimulus-response associations into the behavioral repertoire, especially if the association was acquired during parturition, might, for heuristic purposes, be viewed as an instance of very rapid learning. If the stimulus- response associations are learned, how shall we view the role of the hormonal milieu in this learning process? The hormones might be (a) activating specific neural circuitry by sensitizing the female to a specific range of olfactory, visual, and auditory stimuli, such as those relating to pups and placenta, as Moltz suggested [21], or (b) having a nonspecific effect on the brain that would result in a generalized enhancement of association-formation, the generality of which might have heretofore escaped notice because of the preponderance of parturition-related stimuli, and therefore of parturition-related stimulus-response associations.
That hormones (e.g., those of the pituitary-adrenal axis) can affect classical [10] and avoidance [8,33] conditioning has already been established. The emphasis of the present study is to examine the role of reproductive condition and the entire milieu interne associated with reproductive conditions in conditionability.
Only two studies in the literature appear to have utilized pregnancy, rather than specific hormone treatment, as an independent variable. Banerjee [1] examined learning of a pole-climb conditioned avoidance response in early pregnant, pseudopregnant, and hormonally-treated female rats, and in hormonally-treated male rats. Groups were tested and compared in such a way, however, that although certain statements about the effects of some specific hormones could be made, no comprehensive conclusions about the relationship of pregnancy to learning could be drawn. Nemtsova, Morachevskaia and Andreyeva [24] examined the changes in characteristics of classically-conditioned reflexes at various stages of pregnancy in dogs and rats. Unfortunately, the small number of subjects used and the absence of quantified and statistically-analyzed data, make the meaning of the results entirely unclear to us.
A learning paradigm that could be used to test for generalized facilitation of learning in early pregnant, late pregnant, and nonpregnant rats, had to meet several requirements: (a) the test could not involve cues or reinforcements that related to the parturitional environment (pups, nesting material, placenta, etc.) since these might bias the test in favor of the late-pregnant rats; (b) the test could not utilize food or water rewards, since food and water intake increase dramatically during pregnancy [6,20]; (c) the test could not produce excessive stress which, in turn, might lead to abortion or re sorption of the fetuses; (d) the test could not require demanding locomotor responses which might bias the test in favor of females not gestating near-term fetuses; and (e) the testing procedure should be completable in one day.
The paradigm initially selected was second-order conditioning during pregnancy, superimposed on a previously acquired bar-press shock avoidance. Although bar-press shock avoidance is known to be a difficult task for a rat [2,25], a relatively efficient level of performance was reported to be acquirable with proper shaping and stimulus presentation [3,7,13,17]. We found, however, that the performance of our groups was so far below that predicted in the literature that we were unable to proceed to the secondary conditioning phase. We concluded that other paradigms would have to be used.
EXPERIMENT 1
The first experiment was conducted using a water-maze escape [12,34] that featured two reversals as well as the initial right-left response learning. The water-maze escape, in addition to being a rapidly acquired response, has the added advantage of depending on an easy locomotor task for rodents (swimming), which can be performed even by rodents with severe motor deficits produced by genetic neurological mutations [9].
METHOD
Animals
Thirty Long-Evans (hooded) female rats (Charles River Breeding Laboratories), approximately 5 months old, were tested. The colony was maintained on a 12 hr on/12 hr off light cycle, the on phase beginning at 8:00 am. (EST).
Females were housed individually in a 24 x 19 x 18 cm wire-mesh cage until two days prior to testing, at which time they were placed in a 45 x 19 x 25 cm plastic cage containing 3 cm of sawdust. Food (Charles River Rat/Mouse/Hamster Formula) and water were available ad lib.
Apparatus
The maze consisted of a modified version of that de scribed by Waller et al. [34], which was a topless and bottomless sheet-metal T-maze, with arms curving back toward the start compartment so that the escape ramp cannot be seen from the choice-point. The start compartment, a 25 cm square chamber, opened into a 12 cm alley that was 15 cm long from the start chamber to the choice point. The distance from the choice point to the end of each arm was 72 cm. The hardware-cloth escape ramp shortened the swimming distance in the escape arm by about 10 cm. One arm of the maze was white and the other black; the central alley was gray. The maze, 44 cm high, was placed in water 28 cm deep, a depth sufficient to force the rats to swim. The water temperature was kept at 21°-22.5°C. An aquarium filter/aerator was used to keep the water in the tub from becoming stagnant. Solid waste was removed from the tub after each rat's series of trials. Room temperature was maintained between 24° and 28°C. Between trials, the rat was kept in a light- bulb-warmed plastic cage. The room in which testing was conducted was illuminated by a single 250 W bulb l0 ft above the maze.
Procedure
Each animal was time-bred with a proven male. When an animal was determined to be pregnant (by the presence of a sperm plug or the presence of sperm in the vaginal smear) she was randomly assigned to a group that was to be tested on Day 12 of pregnancy (Midpregnant Group), on Day 22 of pregnancy (Prepartum), or on the first day after delivery (Postpartum). One Midpregnant-group rat was found not to be pregnant, reducing the overall number of animals to 29. Furthermore, some animals were not tested on the critical day and were carried over into the next group (e.g., if a Day 22 animal gave birth earlier than expected, she was tested as a Postpartum rather than a Prepartum animal). The result was an uneven distribution of animals in the three groups (Table 1).
Testing was conducted between 8:00 p.m. and midnight. Each animal was brought into the testing room and tested under all three conditions: initial learning, the first reversal, and the second reversal. For half of the animals, the white arm of the maze was on the right, and for half the white arm was on the left. When the rat had made a turn at the choice point during the first trial, the ramp was placed into the arm that the rat had not chosen, making the first choice always wrong. Criterion for initial learning was 10 consecutive correct (errorless) escape trials. If an error was committed, the error was scored but the rat was allowed to self-correct. Rats rarely turned back into the start alley after an error was committed at the choice point; therefore, the rats almost never made more than one error per trial. Latency to escape was ignored since a speed measure was likely to reflect performance rather than learning differences among the groups. The intertrial interval was 1 min.
After criterion was reached in the initial learning phase, the first reversal occurred, and whichever turn had been correct now became the wrong choice. Criterion for the first reversal was 5 consecutive errorless trials. After criterion on the first reversal was reached, the choice was changed back to the original one for the second reversal; criterion was again 5 consecutive errorless trials. Two Midpregnant and two Prepartum rats stopped swimming during the second reversal phase and were removed from the experiment; this produced a smaller number of animals in the second reversal than in the first. After completion of the test, the pregnant rats were returned to their home cages and were allowed to give birth to test the accuracy of the time breeding and to assess possible harmful effects of experimental manipulations during pregnancy.
RESULTS AND DISCUSSION
Each phase of the task (initial learning, first reversal, second reversal) was analyzed separately in a one-way unweighted means analysis of variance (ANOVA), in which there were three treatment conditions (Midpregnant, Prepartum, Postpartum). Separate analyses were run because (a) the 3 phases comprised 2 different performance criteria, and (b) the total number of animals run under each phase could be included in the analysis.
Reproductive condition had no effect on any of the three phases of the water-maze escape task (Table 1), the F's being nonsignificant (p's > 0.10). To check for a possible condition-by-phase interaction, a subsequent 3 x 3 unweighted means ANOVA for repeated measures was run, using only those rats that completed all phases of testing. The group sizes were those reported in Table 1 for Second Reversal. The main effects of condition and phase were not significant; the F for the interaction, although close, was not significant, F(4,44) = 2.536; p =0.0513. Even if the interaction had been significant, however, the pattern of means (Prepartum and Postpartum doing worse than Midpregnant on the second reversal) is opposite that which would have supported an hypothesis of facilitated learning in periparturient rats.
TABLE 1
NUMBER OF TRIALS TO CRITERION OF FEMALE RATS IN THREE
REPRODUCTIVE
CONDITIONS IN A WATER MAZE ESCAPE
|
|
|
|
||
|
|
|
|
||
| Initial Learning* | ||||
|
|
|
|
|
|
|
|
|
|
|
|
| First Reversal† | ||||
|
|
|
|
|
|
|
|
|
|
|
|
| Second Reversal† | ||||
|
|
|
|
|
|
|
|
|
|
|
|
*criterion = 10 consecutive errorless trials
†criterion = 5 consecutive errorless trials
The possibility exists that the task was too easy and that a ceiling effect made it possible to detect decreases but not increases in learning ability, particularly during initial learning. Notice in Table 1 that the best group had a score of only one more than minimum, and the worse group had a score of less than three more than the minimum. It was decided, therefore, to test the effects of reproductive condition on learning a second time, with the following changes: (a) the use of a paradigm that clearly allowed for detection of in creases in learning ability; (b) the inclusion of groups of non-pregnant females; and (c) the inclusion of a means of testing for retention of the learned association, as well as acquisition.
EXPERIMENT 2
The paradigm chosen for the second experiment was an unsignalled shuttlebox avoidance (modified Sidman avoidance) which had been reported to be rapidly acquired and efficiently performed [26]. We felt that such a task might be difficult enough to allow for the detection of learning facilitation, but not so difficult that the number of shocks taken would lead to premature pregnancy termination. Furthermore, we felt that the response-shock interval we used (17 sec) afforded the rat sufficient time to respond even if burdened by a litter of large fetuses.
METHOD
Animals
Sixty Long-Evans females (Charles River Breeding Laboratories), 60-75 days of age, were used in the experiment. Lighting, caging, and feeding conditions were identical to those in Experiment 1, except that (a) each animal spent 10 days prior to testing in a plastic cage, and (b) each animal spent the last 15 days prior to retesting in a metal cage. The elimination of animals because of failure to cycle, to become pregnant, or to carry fetuses to term, or because of an unexpected solution (such as by body position) to the problem of being shocked (the most common reason) sharply reduced the number of animals. The number of rats completing the acquisition phase of the study was 27, of which 22 went on to complete the retest phase.
Apparatus
Three clear Plexiglas shuttleboxes, measuring 20 x 40 x 20 cm, and having a 2 cm high partition separating the two halves, were used. The boxes were designed so that only the floor tilted on a fulcrum. The floor consisted of 18 3 mm dia. steel rods, approximately 2 cm apart. Each shuttlebox was mounted in a 54 x 40 x 32 cm, sound-insulated chamber (Lehigh Valley), fitted with a blower that exhausted and circulated the air, and also provided a constant background noise. The interior of the chamber was illuminated by a centrally located 10 W bulb.
A Grason-Stadler E6070B shock generator delivered scrambled shocks (0.5 mA, 50 msec pulses, every 2.0 sec) to the side of the box containing the rat, beginning 17 sec after the rat arrived on that side. Crossing as infrequently as once every 17 sec would therefore lead to complete avoidance of the shock.
Procedure
The experiment was conducted in three phases: Pretest, Test, and Retest.
Pretest: Upon arrival in the laboratory, the rats were allowed a week to acclimate to the laboratory conditions, after which a daily vaginal smear was obtained from each female for 1-3 weeks to verify normal estrous cyclicity. After verification, when the rat was in diestrus, the Pretest was conducted consisting of a single 20 min period in the shuttlebox. The number of shocks delivered for each of the 4 five-min blocks of Pretest was tallied and the slope indicating the rate of improvement over the four blocks was computed, which was relatively independent of the absolute level of performance or activity.
Test: The fast- and slow-improvers were evenly assigned to the following 5 groups on the basis of this Pretest score: (1) a nonpregnant group to be tested in estrus (Estrus); (2) a nonpregnant group to be tested in diestrus (Diestrus); (3) a group to be tested on Day 12 of pregnancy (Midpregnant); (4) a group to be tested on Day 21 of pregnancy (Prepartum); and (5) a group to be tested on the first postpartum day (Postpartum).
A 40-day (± 2) period elapsed between Pretest and Test. Ten days before Test, each rat was switched from a metal to a plastic cage (in which the pregnant rats had to deliver). By scheduling back from the projected day of testing, rats that were to be impregnated were time-bred either 2 days, 11 days, or 14 days before being transferred to the plastic cage. This enabled us to keep the time between Pretest and Test and the time housed in plastic prior to Test constant.
On the day of Test, each animal was given a single 50 min run, between 10:00 a.m. and 1:00 p.m., and then returned to her home cage. Pups were removed from the mothers' cages approximately 24 hr after delivery (after Test, in the case of the Postpartum Group).
Retest: Thirty days (± 1) elapsed between the day of Test and the day of Retest. Each animal was transferred back to a metal cage 15 days prior to Retest. Daily vaginal smears were obtained beginning 25 days after the day of Test, and Retest was conducted on a day of diestrus that most closely coincided with the thirtieth day after the day of Test. Retest consisted of one 20 min period.
RESULTS AND DISCUSSION
A program called MULTIVARIANCE [14] was used to provide both multivariate and univariate analyses of variance on only those rats that completed 3 phases of the testing procedure. The use of a multivariate analysis, and therefore the conceptualization of shocks and responses as two characteristics of one dependent variable, allowed for such distinctions as that between rats taking few shocks while making few responses, and those that took few shocks by continuously responding. For purposes of analysis and comparability, the Test phase was separated into T1 (the first four 5 min blocks) and T2 (the last four 5 min blocks); the middle two 5 min periods were disregarded. This provided for 4 data points for each of the 4 phases (Pretest, T1, T2 and Retest). Two MULTIVARIANCE analyses were run. The data used for one run were the total number of shocks and total number of responses per 5 min period, and the data used for the second run were the slope of shocks and the slope of responses across 5 min periods. The analyses could not accommodate missing data in repeated-measures designs, so only animals completing all phases of testing were included. For extraneous reasons (failure to resume cycling, illness, etc.), missing Retest data reduced the n's in the MULTIVARIANCE runs to the following: Diestrus, 6; Estrus, 5; Midpregnant, 4; Prepartum, 4; Postpartum, 3. These analyses were supplemented by additional MULTIVARIANCE runs for analysis of the results of those phases (Pretest, Test) which all rats completed; for these latter analyses, n's were: Diestrus, 7; Estrus, 5 Midpregnant, 6; Prepartum, 4; Postpartum, 5.
The mean number of shocks and mean number of responses per 5 min block in Pretest, Test, and Retest, for each of the 5 groups are presented in Fig. 1.
TABLE 2FIG. 1. Mean Shocks and Mean Responses per 5 min block per reproductive condition, across phases.
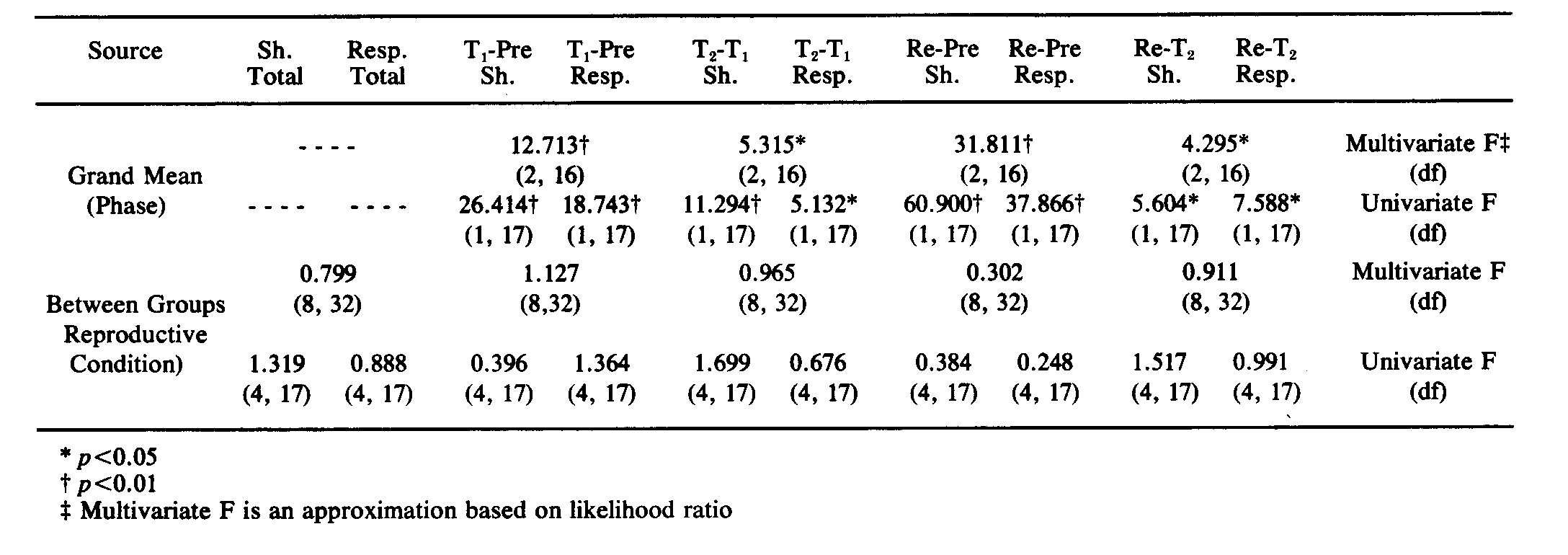
It is clear from Fig. 1 that learning occurred. The multivariate analysis of the total number of shocks and total number of responses per 5 min block for each phase confirmed this. Table 2 contains the multivariate and univariate F's computed by the MULTIVARIANCE program. Notice that learning occurred across all phases of testing. For example, when Pretest was compared with T1, the multivariate F (approximated from likelihood ratio) showed that there was a significant change in the vector (p < 0.00l). The univariate F values calculated by the MULTIVARIANCE program for the change in shocks and the change in responses in the same comparison, indicated that shocks decreased significantly (p < 0.001).
It is, of course, not surprising that learning occurred. The theoretically significant question, however, was whether reproductive conditions had a differential effect on learning. The multivariate F for the Between Groups effect, which indicated the extent to which reproductive condition influenced the change in the dependent variable (vector of number of shocks/block and number of responses/block) revealed that there was no significant influence of reproductive condition. Only one of the five Between Groups multivariate F values (T1-Pre, p > 0.35) even exceeded unity (see Table 2).
The second MULTIVARIANCE run used the slope of shocks and the slope of responses as components of the dependent-variable vector, deemphasizing differences in base rate of responding or in activity level. Three of the four phase comparisons yielded significant multivariate F values. Inspection of the corresponding univariate F's revealed that these were attributable to significant changes associated only with shock. This indicates that although the number of responses made changed significantly across phases of the experiment, the rate of change was constant across phases of the experiment. Furthermore, the analysis using slopes indicated, as did the analysis on totals, that there was no significant effect of reproductive condition on the dependent variable. None of the Between Groups multivariate F values exceeded unity; of the two univariate F's that exceeded unity, the probability associated with the larger F was very high (p > 0.25).
The supplementary MULTIVARIANCE analyses on both the totals and the slopes of shocks and responses for those 27 rats that completed Test but not Retest likewise indicated that whereas there was a significant effect of phases (T1-Pre) for all groups, there was no effect of group (reproductive condition). The results were similar for multivariate F's computed on the slopes of shocks and responses. None of the univariate F's (total shocks, total responses, slope of shocks, slope of responses) were significant. The analyses indicate that, at least for the acquisition phase of the paradigm (Test), exclusion from the original MULTIVARIANCE analyses of the animals that did not complete the Retest did not alter the result. We are confident, therefore, that despite the fact that the MULTIVARIANCE runs included one cell (Postpartum-Retest) in which the n dropped to 3 rats (12 data points), reproductive condition had no effect on either the acquisition or the retention of the task.
One could ask whether the water-maze escape and the unsignalled shuttlebox avoidance, both depending on the removal of an aversive stimulus, are representative enough tasks in an investigation of general learning facilitation. That question may not be directly answerable. It is arguable whether any two tasks can be truly representative learning paradigms, and that only by using an infinite number of tasks can a general facilitation be investigated. We opted to use only two tasks that rigidly conformed to the paradigm- selection criteria we set for the task, rather than to use more tasks, some of which violated the selection criteria. Furthermore, we reasoned that if factors such as motivation and performance bias were carefully controlled, independent variables that produce general learning facilitation should have, to some degree, influenced learning in almost any task we would have chosen.
If a general facilitation of learning does not account for the rapid
development of the components of maternal behavior in the naive parturient
rat, what mechanisms are left that can? One possibility is specific
learning
facilitation, which might be due to an enhancement of associations involving
particular modes of response, or those involving a specific range of stimuli,
or both. These of course might, in turn, be due to changes in motivation,
changes in sensory thresholds, or changes in the threshold for excitability
of central neural circuits that form the substrate for maternal behavior
patterns. Moltz has even suggested [21] that the excitability thresholds
for these neural circuits, once lowered during the first parturition, may
remain low permanently, thus accounting for the ease with which external
stimuli elicit maternal behavior responses in parturitionally experienced
rats. Whether this circuitry is hard-wired or is the result of acquired
functional connection that depend on certain develop mental experiences,
is quite another matter. The answer to these questions about specific mechanisms
are beyond the scope of the present study, and may, in fact, not be presently
directly testable. We believe, though, that we may have taken the first
step toward elucidating the mechanism, not by specifying what the mechanism
is, but rather by specifying what the mechanism is not. It is not, apparently,
a general facilitation of learning ability.
1This research was supported by NSF Grant BNS
76-04316, by NIMH Grant MH25747, and by Institutional Funds from SUNY/Buffalo,
all awarded to M.B.K.
2Address reprint requests to: M. B. Kristal,
Department of Psychology, SUNYAB, 4230 Ridge Lea Road, Buffalo, NY 14226.
REFERENCES