The post A puzzle from the past appeared first on Exploring our Oceans .
]]>
South England’s coastline features chalk cliffs. Chalk is made of skeletons of coccoliths. They sank down on the seafloor from the sunlit waters above. The 100 meters cliff shows a 100 million years history – peaceful deposition during the Cretaceous followed by dramatic movement of land and sea.
While hiking in Lake District, views are different. Mountains consist of igneous rocks that are no longer white. As a result, we may find the drinking water not that ‘hard’. As we move on, stop by a giant rock, and stare at the scratches, a picture of glacier just appears.
Further north of the land, we may be impressed by the dark basalts. They are formed from cooling of lava, related to Cenozoic volcanic activities when the opening of the Atlantic began.
Geochemists are using elemental and isotopic tools to decode the clues about the Earth’s history. For example, the rise in atmosphere oxygen level will mobilise redox-sensitive elements (like iron and chromium), and will change their isotopic compositions. The delicate variations in metal isotope signatures that are recovered from sedimentary rocks are hints of the past climate change.

The power of nature not only travels in time, but also shapes nowadays landscapes. I thought of a field trip to a small island off the coast, which is characterised by muddy flat on its west coast while sandy beach on its east. Under weak hydrodynamic condition, fine sediments deposit, and form the salt marsh. On the other side, contrastively, strong currents bring the coarse sands, as well as tourism.
When human activity adds onto the natural power, things become more complicated. Once during my master’s project, I surveyed an estuarine area where metal rich effluents were discharged from industries. You know, however, estuary is the sensitive zone that links land and sea and hosts living communities. Hopefully I can see the area is now healing from lack of mitigation,
‘Things will settle back to their original rhythms, season after season’.
We’re now stepping into Anthropocene, a proposed geological epoch dating from the commencement of significant human impact on Earth’s geology and ecosystems. This is an epoch that our actions can positively shape the future of our blue planet.
The post A puzzle from the past appeared first on Exploring our Oceans .
]]>The post Hot vents, cool people appeared first on Exploring our Oceans .
]]>As you’ve seen in Week 1 of the course, hydrothermal venting occurs when seawater penetrates into the ocean crust, becomes heated, reacts with the surrounding rock, and then rises to the seafloor as fluid and gas. Forty years of exploration has yielded an inventory of more than 500 active vent fields (according to Baker et al., 2016). They are often thousands of meters below the surface of the ocean, along the large volcanic mountain ranges called the mid-ocean ridges.

These submarine hot springs are a major gateway for the exchange of heat and chemicals between the solid earth and the deep ocean. The chemicals coming out of the hydrothermal vents could be liquids, particles or gases, and include inorganic compounds as well as organic molecules. The leaky vents play a potentially important role in the cycling of these chemicals in the oceanic inventory.
You have noticed the fascinating life in hydrothermal vent ecosystems – can you imagine how these organisms survive in the high pressure, high temperature, and toxic environments? They don’t even need sunlight, as chemical energies are supplied through a process called chemosynthesis. These also provide insights into origins of life on Earth.

In the year 1985, a group of scientists from Cambridge participated in a cruise that produced the first photographs of a hydrothermal vent called the TAG (Trans-Atlantic Geotraverse) which is the first vent discovered on the Mid-Atlantic Ridge. The spreading rate of the Mid-Atlantic Ridge is slow (less than 40 mm/yr), and this means such hydrothermal phenomena is just not limited to fast-spreading oceanic ridges (Rona et al., 1986).
In the 1990s, a multidisciplinary scientific investigation of the mid-ocean ridges was conducted by a list of British institutes (BRIDGE Programme, https://wikipedia.org/wiki/British_Mid-Ocean_Ridge_Initiative). Many scientists and research centres around the UK contributed to this programme, and cruises explored the North Atlantic, Southwest Atlantic, and Southwest Pacific. ”Every area- geophysics, geochemistry, biology and technology, had success.” But does this mean the hydrothermal exploration is complete? Not really. There are still questions to answer- about how many hydrothermal vents, about organisms that surround the vents, about the chemical fluxes transported to the oceans.
Here is a link from NOC website showing the research ships, and you must have been aware that they are the primary method of oceanographic observation http://noc.ac.uk/facilities/ships Each research expedition has a cruise ID (for example, DY is for Discovery and JC for James Cook). The maiden scientific voyage of James Cook was in 2007, and after 12 years, the JC180 expedition has now finished. Behind these numbers, it was the scientific missions that have been achieved. The missions have always considered hydrothermal exploration as important. I was impressed when I was exploring the inventory of research cruises onboard James Cook, here I’d like to list some of what I’ve read:

JC042 (2010-01-07 to 2010-02-21): the exploration of deep-sea vents around Antarctica;
JC044 (2010-03-25 to 2010-04-22): the discovery of the world’s deepest hydrothermal vent in the Cayman trough;
JC080 (2012-12-02 to 2012-12-30): revisiting Southern Ocean vents that teem with life http://hotventscoldocean.noc.ac.uk/;
JC082 (2013-02-06 to 2013-03-08): revisiting hydrothermal vents in the Caribbean http://intothecaymanabyss.noc.ac.uk/
JC138 (2016-07-08 to 2016-08-24): exploring seafloor massive sulphide deposits around TAG https://bluemining.eu/research-cruise-2-james-cook-138/
JC156 (2017-12-20 to 2018-02-03): exploring iron supply from Mid-Atlantic Ridge https://ga13fridge.wordpress.com/
……
The vents are hot, the sciences (and people who are devoted to sciences) are cool. What’s going to be the next expedition?
References:
Baker, E.T., Resing, J.A., Haymon, R.M., Tunnicliffe, V., Lavelle, J.W., Martinez, F., Ferrini, V., Walker, S.L. and Nakamura, K., 2016. How many vent fields? New estimates of vent field populations on ocean ridges from precise mapping of hydrothermal discharge locations. Earth and Planetary Science Letters, 449, 186-196.
Deamer, D., 2014. Origin of life: The first spark. Nature, 514 (7522), 302.
Rona, P.A., Klinkhammer, G., Nelsen, T.A., Trefry, J.H. and Elderfield, H., 1986. Black smokers, massive sulphides and vent biota at the Mid-Atlantic Ridge. Nature, 321 (6065), 33.
The post Hot vents, cool people appeared first on Exploring our Oceans .
]]>The post Trace metal in the ocean: less is more appeared first on Exploring our Oceans .
]]>One billion litres of seawater would be required to gather only 25 grams of iron. However, this element is essential to every form of life on the planet. ‘Give me half a tanker of iron and I’ll give you the next ice age’ – this is what the iron hypothesis (Martin, 1990) would expect. The adding of micronutrient iron to some parts of the oceans (see the figure below) can stimulate the growth of phytoplankton, and may cure the global warming. Although the consequence of iron fertilization is not yet clear, we indeed realised the significance of trace metals as many of them are critical for marine life and therefore influence the functioning of ocean ecosystems and the global carbon cycle.
Global distribution of sea-surface chlorophyll levels. As a proxy for phytoplankton mass, chlorophyll is relatively low in North Pacific, Equatorial Pacific, and Southern Ocean, yet nutrients are available in these three regions. Image credit: Wikipedia
The next question is, in the oceans, where do the trace metals come from and where are they going? The weathering process initially provides both dissolved and particulate forms of the metals through riverine input. Dust deposition contributes to the inventory, so does sediment input from the other way round – see how important the ocean boundaries are. When I was an undergraduate student 5 years ago, the text book told me hydrothermal iron delivered to the oceans could be neglected, but this idea has now been challenged, and the deep leaky vents have become the hotspots.
The simplified oceanic iron cycle from a review paper. The major external source is dust, with the iron supplied from continental margins and hydrothermal activity on mid-ocean ridges. However, this paper does emphasise that this schematic is not up-to date now because of our improved understanding about the sources and internal cycling of trace elements in the ocean. Image credit: Tagliabue et al. (2017)
If you compare iron data from 1960s onwards and you find a decrease in concentrations through time, it may not reflect the real situation as such element is contamination sensitive considering its scarce amount in seawater. Moreover, to measure trace elements at large time and space scale is clearly required for us better knowing the distributions of these elements and the processes behind. I went onto a GEOTRACES expedition to the North Atlantic, and GEOTRACES is a global collaboration of oceanographers seeking to find the chemistry clues. The clues are about, trace metal and isotopes, and during the past 15 years contributed by 35 nations, more than 100 research cruises have been completed under the GEOTRACES scheme. We look forward to hearing more stories about the ocean chemistry, especially about the role of trace elements in the past and at present, and the impact on the future.
In the map, yellow lines represent completed cruises, red lines are planned expeditions and black are cruises completed as part of a collaboration with other research programs. Image credit: GEOTRACES
References
Martin, J.H., 1990. Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography, 5, 1-13.
Tagliabue, A., Bowie, A. R., Boyd, P. W., Buck, K. N., Johnson, K. S., Saito, M. A., 2017. The integral role of iron in ocean biogeochemistry. Nature, 543(7643), 51.
The post Trace metal in the ocean: less is more appeared first on Exploring our Oceans .
]]>The post How far are we from the deep ocean? appeared first on Exploring our Oceans .
]]>Lt. Don Walsh and Jacques Piccard in the bathyscaphe Trieste, making the first descent to the Challenger Deep in 1960. Image credit: Steve Nicklas, NOAA Ship Collection.
What I wanted to tell first is that the deep ocean is never an isolated system. During the MOOC course you’ve explored the large-scale thermohaline circulation wherein the denser and colder deep seawater plays a vital role. If something goes wrong in this loop, such as unusual melting of Greenland ice sheet with excessive production of fresh water – you know this has happened in the geological past, the global circulation as well as the heat transport could be disrupted. Subsequently such disruption would cause changes to the whole climate system in the world. Increased in-situ observations for the thermohaline circulation as well as improved numerical modelling thanks to super computers are now undertaken by researchers, aiming to clarify the physical mechanisms behind and to provide predictability for the climate.

Circulation patterns in the North Atlantic Ocean. Cold, dense seawater is shown in blue, flowing south from high latitudes, while warm and less dense water is shown in red, flowing north from low latitudes. Image credit: Jack Cook, for Ocean and Climate Change Institute, Woods Hole Oceanographic Institution.
You may also be surprised by a variety of hydrothermal vents that lie in the deep ocean, especially on mid-ocean ridges. The vent fluids emitted from seafloor generally contain extremely high concentrations of trace elements such as iron, manganese, copper, zinc… For a long time this source of trace elements to the oceans has been overlooked as people considered most of them would have been precipitated and settled down within the first few seconds of hydrothermal venting. However, up-to-date researches illustrate long distance transport of iron and some other trace elements away from the original vent fields into the ocean, which makes us have to reconsider what is going on between these ‘hot ports’ and the deep seawater. An interdisciplinary study is clearly required, and probably you’ve heard about the word ‘biogeochemistry’ which has become a very popular topic during the past 10 years and will continuously be focused on by people across the world, including me  All in all, it would be amazing if we can find hydrothermal sourced iron in intermediate and even shallow parts of the ocean, because this means the iron from the deep can possibly fuel the primary productivity in the surface, which will ultimately have an impact on the global change that we care about.
All in all, it would be amazing if we can find hydrothermal sourced iron in intermediate and even shallow parts of the ocean, because this means the iron from the deep can possibly fuel the primary productivity in the surface, which will ultimately have an impact on the global change that we care about.
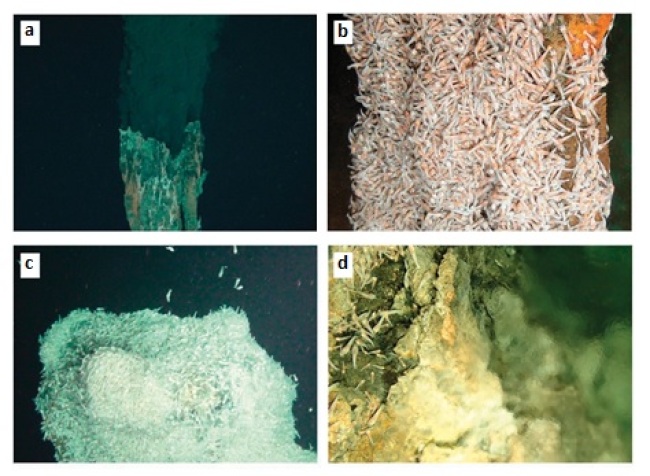
Chimneys and fauna from the Mid-Cayman Spreading Centre. (a) High-temperature venting at the Beebe vent field, depth 4960 m. (b) Aggregation of alvinocaridid shrimp on an active chimney at the Beebe vent field. (c) Peak of sulphide edifice at the Von Damm vent field, depth 2300 m, covered by aggregation of alvinocaridid shrimp. (d) Clear vent fluids and alvinocaridid shrimp at the Von Damm vent field. Image credit: Connelly, D. P. et al. Nat. Commun. 3:620 doi: 10.1038/ncomms1636 (2012).
These hydrothermal vents not only contribute chemical components to the oceans, but also serve as habitats for a diversity of biological activities. Please bear in mind that at the same time the vent fluids are enriched in sulfide and other reduced compounds (CH4 and H2), providing a source of chemical energy that can specially support life through chemosynthesis rather than photosynthesis. The bacterial community utilize such energy to generate organic matter (e.g. carbohydrate), supporting a high faunal biomass and providing nutrition for macroinvertebrates through symbiotic association. The figures above (taken from RRS JC44 cruise) just show the fantastic faunal observations from the Mid-Cayman Spreading Centre.
The adaption of eyes due to changes in lights was discussed in the course (week 3), and you also learnt that pressure is another variable that will affect life in the deep ocean. During a recent research cruise (RRS JC156) to the North Atlantic Ocean, we sent the polyethylene cups together with our water sample to as deep as 4000 meters – see what the cups looked like when they were back. Then you may realize how hard marine organisms protect themselves from being condensed. I would say, a promising approach for us to investigate the adaption mechanisms is to compare the genetic differences between life in deep sea and in shallow water.

Sending the cups down to 4000 meters – see what they look like when they are back!
The deep ocean is tightly associated with our daily life, but as many of you have pointed out, the exploitation of resources above or beneath the seafloor should be done with dual caution. It is necessary to assess any potential risks rising from what human beings interact with the blue planet, for example, the action of sub-seabed CO2 storage. It would be amazing if the capture of CO2 from the atmosphere and the stabilization into the ocean sediments work well, as this marks we are able to ‘moderate’ the climate given the global warming is driven by increased CO2 level. However, what if the CO2 storage site leaks? What’s the potential influence on benthic organisms and marine ecosystem? We need to answer these questions. Hopefully, a group of scientists are working together to develop comprehensive monitoring strategies and to set up guidelines for the best environmental practices. In addition, the National Oceanography Centre and the University of Southampton have collaborated for years in improving autonomy and generating advances in marine robotics (e.g. autonomous underwater vehicle, know as AUV), which technically enhances out confidences in addressing the questions above.

Autosub 6000, one of the National Oceanography Centre’s AUVs. Image credit: Doug Connelly, http://www.stemm-ccs.eu.
There is still a long way to go, but we are on the way getting our dreams approached!
The post How far are we from the deep ocean? appeared first on Exploring our Oceans .
]]>The post Adventures of Clair Patterson appeared first on Exploring our Oceans .
]]>The first task for Patterson was to measure the concentration and isotope composition of lead inside the zircon, which is extremely useful for geological dating. The lead present in zircon indicates the decay from uranium, appearing to serve as an accurate clock (details see: https://en.wikipedia.org/wiki/Uranium-lead_dating). Moreover, if the composition of the world’s primordial lead (e.g. from iron meteorites, as origins of the Earth and meteorites should be related) can be figured out, scientists are supposed to tell the Earth’s age!
Iron meteorite found in Canyon Diablo (figure credit: Geoffrey Notkin)
This was a brilliant idea however followed by tough laboratory work. It was found that no matter how crazy Patterson measured the concentration and isotope composition of lead in the zircon sample, disappointing results always showed up – exceeding values with poor reproducibility. Then, it was realized that there must be some lead coming from outside the lab or even the atmosphere that contaminated the samples and ruined nearly all the experiments!
To this end, Patterson began to clean his laboratory by hand, trying to wipe all the lead away from the working area. It wasn’t in vain, but still not much improved…… Things turned out to be better until Patterson and his supervisor, Harrison Brown, moved to Caltech where the world’s first ultra-clean room was built up. The hope just came along.
Clair Patterson working at Caltech (Photo credit: Caltech E&S Magazine)
Here by saying ‘ultra-clean’ I mean the air coming to the room is filtered and the dust is excluded – you know dust is dusty. The cleanroom then became a necessary for the metal isotope research across the world’s laboratories (see: https://en.wikipedia.org/wiki/Cleanroom) and to my knowledge it marks the cornerstone of ‘trace-level’ exploration.
One of the ultra-clean rooms at the National Oceanography Centre, University of Southampton
It had been 7 years since the young man began to study the lead, and finally he made it! These were Patterson’s whispers: Thank you Charles Lyell (the author of Principles of Geology)… Thank you Ernest Rutherford (the father of nuclear physics)… Thank you Harrison Brown (dear supervisor)…Thanks to all the scientists who had contributed to field… Thanks to the state-of-the-art analytical technique (multiple collector inductively coupled plasma mass spectrometry, https://en.wikipedia.org/wiki/Isotope-ratio_mass_spectrometry)… Here we go: it is 4.5 billion years – the Earth’s age!!!
The lead-lead isochron for meteorites (Patterson, 1956)
It appears to be a happy ending, however I would say, this is just the beginning. At the time of wiping down the interferences of lead from the laboratory, Patterson began to think about the sources of lead. Afterwards Patterson got the chance to further investigate the vertical distribution of lead in the oceans, and what surprised him was that the concentration of lead in shallow water was hundreds of times greater than in deep ocean… The most contaminated areas were close to the east and west coasts of the United States where the vehicle industry was highly developed… Based on investigations from the polar ice core, the lead level was even hundreds of times greater than the geological past…
An example from RRS JC156 expedition showing 'clean' technique for water sampling at sea
All these facts points a mass ‘poisoning’ on an unprecedented scale, as for many years lead has been known to cause brain damage, development impairment, violent behaviour, and even death. Nonetheless, the addition of tetraethyl lead (https://en.wikipedia.org/wiki/Tetraethyllead) to gasoline as an antiknock agent has become a practice since 1921. Patterson went public with his discoveries about lead, publishing the findings and sending copies to government leaders. He fought against the industry for another 20 years and his efforts accelerated the phaseout of lead from all standard, consumer, automotive gasoline in the US in the 1980s.
Clair Patterson’s story inspired me a lot: the man who figured out the Earth’s age was also responsible for one of the greatest public health victories of the 20th century!
References:
Patterson, C. C. (1956). Age of meteorites and the Earth. Geochim. Cosmochim. Acta 10, 230-237.
deGrasse Tyson, N., Druyan, A., Braga, B., & Pope, B. (2014). Cosmos: A spacetime odyssey. National Geographic Series. Available at: http://channel.nationalgeographic.com/cosmos-a-spacetime-odyssey.
The post Adventures of Clair Patterson appeared first on Exploring our Oceans .
]]>


