Replacement
Publications
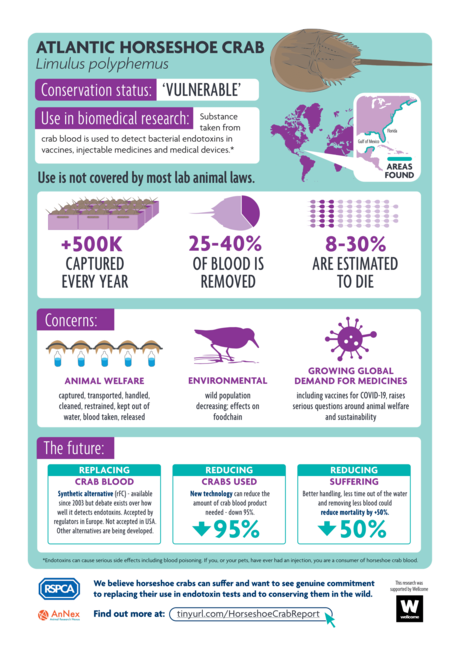
Endotoxin testing is a vital part of quality and safety control in pharmaceutical production. The primary method for this testing in North America and Europe is the limulus amebocyte lysate (LAL) test, a critical component of which is the blood of Atlantic horseshoe crabs (Limulus polyphemus). Procuring blood for LAL testing involves capturing and bleeding over 500,000 crabs from wild marine populations each year. Whilst efforts are made by manufacturers to return crabs to the sea following the collection of blood, there is a level of mortality and sub-lethal impact involved, prompting increasing discussions about welfare and ethics. The 3Rs – the ambition to where possible, replace, reduce, and refine the use of animals – are established and accepted worldwide as the best framework for governing animal-dependent science. However, the biomedical utilization of horseshoe crabs to produce the LAL test has rarely been viewed through a 3Rs framework. More recently, there has been a renewed attention on sustainable methods and alternatives to the LAL test. Drawing on in-depth qualitative interviews, this article examines stakeholder perspectives on opportunities for thinking with the 3Rs, considering current appetites to replace, refine, and reduce contemporary biomedical reliance on horseshoe crabs. The shape of conversations about the biomedical utilization of horseshoe crabs has shifted significantly in recent years, and the 3Rs are an important driver of change, offering the potential to advance the use of more sustainable methods, and realize the welfare considerations increasingly expected across science and society.
Rich Gorman’s secondment to the RSPCA explored the social relations shaping the use of horseshoe crab blood within pharmaceutical endotoxin testing. This involved 13 stakeholder interviews, and has resulted in a stakeholder report alongside a peer-reviewed journal publication. Rich has also presented the research at industry conferences and academic seminars. This is the report and infographic produced for his research.
This report explores what a social science perspective might add to understanding the debates surrounding the use of horseshoe crabs in endotoxin testing. It draws on qualitative research with stakeholders, alongside documentary and policy analysis to examine the various perspectives, positions, and sides of debates about horseshoe crabs. This report attempts to represent the wide diversity of stakeholder perspectives about the biomedical use of horseshoe crabs in a balanced manner.
Blog entry
Rich Gorman’s secondment to the RSPCA explored the social relations shaping the use of horseshoe crab blood within pharmaceutical endotoxin testing.
As part of a Wellcome Trust Secondment Fellowship with the RSPCA's Research Animals Department Dr R
As part of a Wellcome Trust Secondment Fellowship with the RSPCA's Research Animals Department Dr R


.gif%3Fitok=DjRU-WH5)
