Institute of Physiology, Bulgarian Academy of
Sciences, Acad. G. Bonchev str. bl. 23, 1113 Sofia, Bulgaria,
1 Electro-Neuro-Physiology
Research and Application Center, University of Istanbul, 34390 Çapa-Istanbul,
Turkey,
2 Turkish Scientific and Technical
Research Council (TÜBITAK), Brain Dynamics Research Unit, 06100
Kavaklidere-Ankara, Turkey
CA Corresponding Author:
Assoc. Prof. Dr. Tamer Demiralp
Istanbul University
Istanbul Faculty of Medicine
Department of Physiology
34390 Çapa-Istanbul, Turkey
Tel (Fax): 90 212 533 94 68
e-mail: karamusa@hamlin.cc.boun.edu.tr
In the auditory modality, simple tone bursts elicit transient EEG gamma band responses (GBRs) that are strongly phase-locked to stimulus in the first 100-120 ms. Such GBRs can be recorded from various cortical and deep brain structures of animals [6,7] and from the scalp in humans [8,9], which implies that auditory gamma band oscillations may reflect sensory processing. However, no tonotopic presentation of the MEG gamma band field in the auditory cortex has been found [10]. It has also been demonstrated that the power of the early synchronized GBR is larger to attended than to unattended auditory stimuli and is thus associated with selective attention mechanisms in humans [11]. The power of the early GBR also has been related with integrative sensory-motor mechanisms as it is enhanced in subjects who intensively focus their attention to motor response preparation [12]. It is noteworthy that auditory GBRs later than 120 ms also correlate with attention to auditory targets [13] and sensorimotor integration [12,14]. It is not known, however, whether the involvement of early and late GBRs in the processing of attended motor-task stimuli is similar.
One major problem in approaching this question arises from the fact that the early GBRs are well synchronized with stimulus, whereas the late gamma oscillations are weakly phase-coupled with stimulus and can not be detected in averaged potentials [2,10,12]. Furthermore, in the averaged potential, amplitude and phase-locking effects are confounded and cannot be evaluated separately. Usually, only power variations of single GBRs are analyzed. However, the phase-locking of event-related oscillations from other frequency bands (alpha and theta) has been demonstrated to vary with processing conditions independently of amplitude/power alterations [15,16]. Thus, apart from amplitude differences, changes in the stability of phase-locking of GBRs may be suggested to reveal specific functional aspects of the gamma band activity during stimulus processing in task conditions.
The present study was designed to analyze in a systematic
manner the effects of two independent task variables, stimulus certainty
and motor-task relevance, on the early and late auditory gamma responses
in humans. Stimulus certainty was chosen as a variable that can modify
expectancy or focused attention to stimulus. To study motor-task effects,
high- and low-certainty stimuli differed in that they required or not motor
responding. In addition, it was aimed to assess phase-locking independently
of amplitude effects by quantifying the between-sweep synchronization of
event-related gamma oscillations at the level of single sweeps [17].
Thus, the major questions addressed in the present work were: (1)
Do stimulus certainty and motor-task relevance have independent effects
on event-related gamma activity? (2) Are these effects specific for
gamma response amplitude and phase-locking? and (3) Are these effects specific
for early and late gamma band responses?
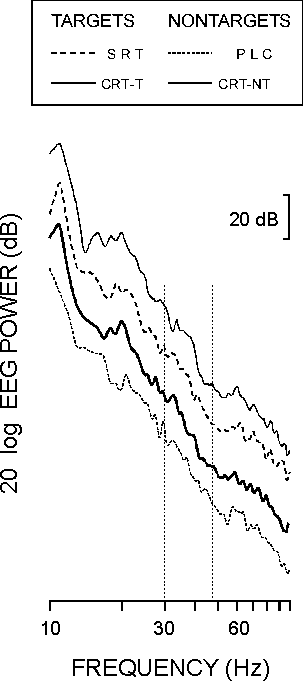
The cut-off frequencies of the EEG amplifiers were 0.1 and 120 Hz. Analysis epochs of 1024 ms before and 1024 ms after stimulus were sampled with a frequency of 250 Hz. Any EEG or EOG trial exceeding ±50 µV was excluded from further analysis and 40-42 artifact-free sweeps of each stimulus type were used. Event-related power spectra were computed for the post-stimulus epochs by means of Fast Fourier Transform (FFT). Figure 1 shows that the spectral power in the higher frequency ranges (60-80 Hz) decreases for each stimulus type, which indicates that no muscle activity is present in the EEG data [18]. Single EEG trials were digitally band-pass filtered with zero phase shift in the gamma frequency range (band width 30-45 Hz, 12 dB/oct, roll-off negative).

| FIG. 1. Power spectra of post-stimulus EEG in the passive listening condition (PLC), simple reaction task (SRT), and choice reaction task targets (CRT-T) and nontargets (CRT-NT). Curves are shifted in order to visualize the monotone decrease in the power spectrum. Frequency range of analysis is marked. |
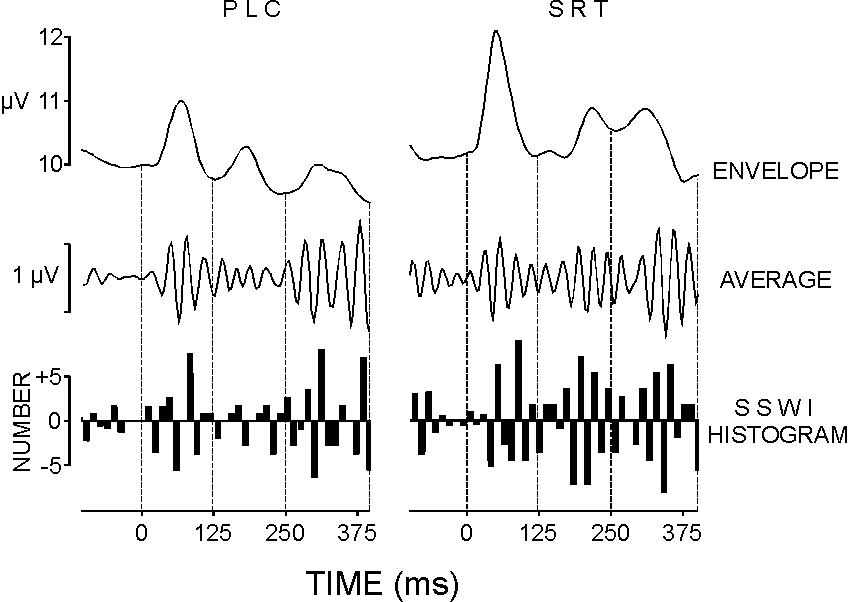
| FIG. 2. Event-related gamma response of one subject at Fz: average upper envelopes, average gamma responses, and SSWI histograms in the passive listening condition (PLC) and simple reaction task (SRT). Time windows used for measurements are marked with dashed lines: 0-120, 120-250, and 250-400 ms. |
For a quantitative evaluation of the phase-locking, a modification of the single sweep wave identification (SSWI) method was applied [17]. Briefly, extremes (minima and maxima) were identified in each filtered (30-45 Hz) single sweep. Maxima were replaced with (+1) and minima with (-1). The latency positions and coded amplitudes were stored. For each 12 ms time interval (nearly one half period of the 40 Hz rhythm), a summation of the coded extrema was performed across trials. The values obtained after summation were assigned to a histogram bar (SSWI histogram). Thereafter, the SSWI histogram was normalized by dividing the bar values to the number of single sweeps included in the analysis. Typical SSWI histograms reflecting the phase-locking of GBRs are illustrated at the bottom of Fig. 2. For statistical analysis, the absolute bar values of the normalized SSWI histogram were summed along the time axis for each time window separately. Thus, information is extracted about the strength of single GBR phase-locking in the successive post-stimulus periods corresponding to the serial gamma bursts. The sums were determined for each subject, stimulus type, and electrode. Similar measurements were made for a reference period in the pre-stimulus epochs. Possible effects of frequency variations on the phase-locking parameter were controlled by measuring and analyzing statistically the mean number of the identified extrema in each time window.
Figure 2 shows an example from the PLC and SRT conditions to demonstrate that the appearance of gamma bursts may be accompanied by either strong or weak phase-locking of the gamma waves within these bursts. In the early (0-120 ms) period, enhanced and phase-locked gamma waves are observed for both stimulus types. Although envelope peaks are seen in the middle (120-250 ms) time windows of both envelope curves, the SSWI histogram of the GBR in PLC shows no phase-locking in this latency range, while a strong phase-locking is evident for the 120-250 ms epoch in SRT. For the 250-400 ms time window, a synchronization of single gamma oscillations is seen in both the PLC and SRT potentials.
Statistical analysis: Single-sweep measures of amplitude
and phase-locking from each time window were subjected to ANOVAs with three
within-subjects factors: task [targets (SRT and CRT-T) vs
non-targets (PLC and CRT-NT)], certainty [high (PLC and SRT) vs
low (CRT-NT and CRT-T)], and electrode (Fz, Cz, and Pz). Maximal peak-to-peak
amplitude of averaged GBRs was also subjected to the same analysis. For
all ANOVAs the degrees of freedom were corrected by using the Greenhouse-Geisser
procedure. The original df and corrected probability values are
presented in the results. The phase-locking of GBRs in each time window
was compared with the level of the pre-stimulus gamma activity phase-locking
by means of non-parametric Wilcoxon-Wilcox test.
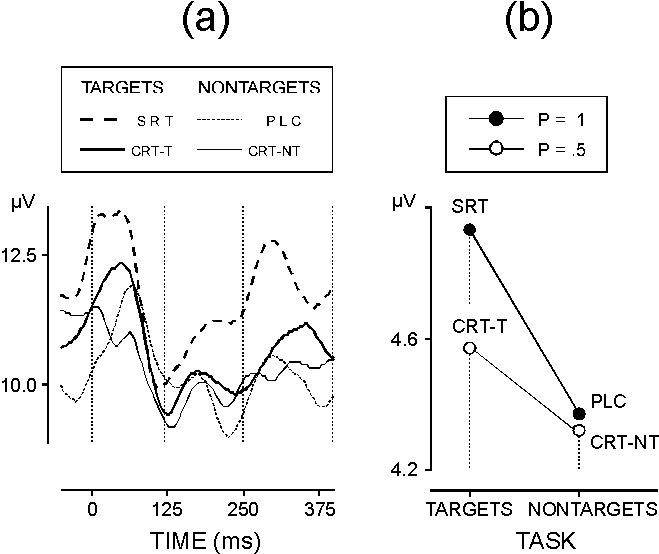
| FIG. 3. (a) Grand average upper envelopes of gamma responses in different experimental conditions (for designations see Fig. 1). (b) The effect of task-relevance x certainty on the amplitudes of single gamma responses in the time window 0-120 ms. Stimulus certainty is high (p = 1) in PLC and SRT, and low (p = 0.5) in CRT-NT and CRT-T. |
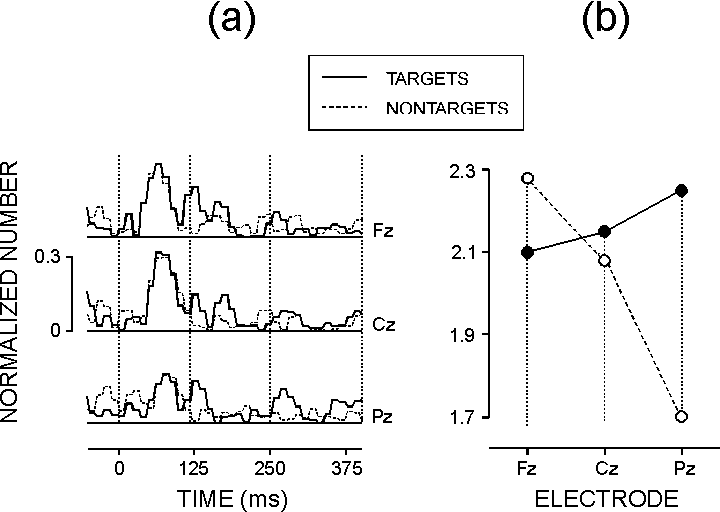
| FIG. 4. (a) Grand means of the rectified normalized SSWI histograms for targets (SRT and CRT-T) and nontargets (PLC and CRT-NT). (b) The effect of task-relevance x electrode on the normalized number of phase-locked single gamma waves in the time window 250-400 ms. |
Early gamma band activity: In addition to the evidence from averaged GBRs [8-10,12,13,20], the separate analysis of phase-locking proved that the early GBR was strongly synchronized with stimulus. Nevertheless, early GBR phase-locking did not differ between the processing conditions related to stimulus certainty and task relevance. By contrast, gamma response amplitude was significantly larger for highly expected (predictable and relevant) motor-task stimuli, which was not evident from average data analysis. Hence, an amplitude increase of the early GBR appears specifically related with high expectancy and focused attention to response preparation. This finding is consistent with previously reported associations of gamma band EEG power with states of high arousal, alertness, and focused attention [12,14,21]. Increased gamma band power has also been observed over localized sites of the primary motor cortex approximately 200 ms before finger movement [18,22], which makes it possible that activity related to motor preparation may have contributed to the present results from the SRT condition (mean RT 240 ms). However, low-certainty targets (mean RT 390 ms) also produced larger amplitudes than did non-targets (Fig. 3a,b). Hence, motor-task stimuli expected with either high or low certainty can enhance the amplitude of the early GBR. This indicates that a focused attention mechanism of controlled processing rather than motor-related activity is likely to have increased the amplitude of the early (phase-locked and non-phase-locked) GBRs. Given the role of cortico-thalamo-cortical systems in the activation of task-specific cortical areas [23], the enhancement of both phase-locked and non-phase-locked early GBRs may be therefore regarded in the context of increased recurrent thalamo-cortical activation involved in the generation of neocortical gamma band activity in man [20,24]. The strong and task-independent phase-locking of the early GBR at the three midline electrodes implies that irrespective of gamma power variations substantiating functionally relevant processes, sets of stable and well defined constraints are tuned to oscillate synchronously in the gamma range at distant cortical locations [20,24], which may subserve the dynamic linkage among distant cortical areas in order to integrate auditory percepts.
Late gamma band activity: In line with previous reports
on averaged data [12,25], the present results show that the late gamma
band activity can be characterized as weakly phase-locked to stimulus.
In spite of this, it is noteworthy that the phase-locking of the late gamma
activity was associated with motor task-relevance. This finding is of interest,
because only amplitude (power) effects have been previously described for
the late gamma band activity in task conditions [4,5,12,13]. The separate
quantification of phase-locking performed here showed that within 120-400
ms after stimulation both predictable and unpredictable targets produced
more strongly phase-locked late GBRs relative to non-targets. It is not
likely that motor programming and execution are responsible for this effect
because of the following reasons: First, motor response preparation
and execution have been shown to affect the power of gamma band activity
[18,22]. In the present study, no amplitude differences related to the
motor task were found for the late GBRs. Second, motor-related activity
should have produced significant task x certainty interactions in each
of the 120-250 and 250-400 ms windows because of the significant difference
in reaction times in the SRT (mean 240 ms) and CRT (mean 390 ms), but no
such interactions were found. Third, the task effect on gamma phase-locking
did not differ between the recording sites for the 120-250 ms period and
was focused to the parietal brain area for the 250-400 ms period. The parieto-temporal
brain areas have been found to be predominantly engaged when high demands
to sensory-cognitive processing are imposed [14]. The possible contribution
of muscle activity to the observed effects can also be ruled out because
a decrease of spectral power was observed for the 60-80 Hz range. In addition,
no significant effect was found for late gamma response amplitudes that
should be most sensitive to possible muscle activity contribution. In contrast,
a significant effect was obtained for the phase-locking that is insensitive
to random or non-locked signals like the EMG. There was also no significant
task x certainty interactions for the amplitude, which was to be expected
if muscle activity has contributed to the recorded gamma activity, due
to the significantly different reaction times. The shifts in topography
effects can not be explained with contamination from EMG. Therefore, the
observed differences in the between-sweep synchronization of gamma responses
may be primarily attributed to the processing demands of the motor task-relevance.
The stronger and site-specific phase-locking of the late gamma response
to targets may therefore reflect a more general cognitive process related
to an endogenous resetting of selected and defined networks during motor-task
performance. It will be an object of a future study to explore whether
it is the motor response integration, the task-relevance per se, or both,
that cause a stronger phase-locking of late gamma responses to targets
relative to non-targets.
Conclusion
The present results indicate that early auditory gamma band responses
appear primarily associated with focused attention, while the late gamma
responses vary with motor-task relevance. The specific functional involvement
of early and late event-related gamma activity was revealed after single
response amplitude and phase-locking were analyzed separately, which may
not be detected in average data. Thus, along with power measures, the stability
of phase-locking of gamma band responses should be regarded as a functionally
meaningful parameter varying with processing demands and recording site.