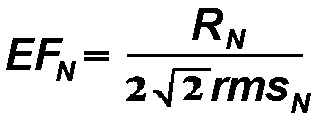Yordanova, J., Kolev, V. (1998). Developmental changes
in the theta response system: a single-sweep analysis. Journal
of Psychophysiology, in press. © 1998 Federation of European Psychophysiology
Societies
Developmental Changes in the Theta Response System:
a Single-Sweep Analysis
Juliana
Yordanova and Vasil
Kolev *
Institute of Physiology, Bulgarian
Academy of Sciences, Sofia, Bulgaria
Accepted for publication: March 13, 1997
*Corresponding author:
Institute of Physiology, Bulgarian Academy
of Sciences,
Acad. G. Bonchev str. bl. 23, 1113 Sofia,
Bulgaria.
Tel.: (359) 2-979-37-49 Fax: (359) 2-738-469
email: kolev@iph.bio.bas.bg
Abstract
Recently, increased interest was focused on the EEG frequency responses
in the theta (4-7 Hz) range because of their association with stimulus
information processing. However, it is not known whether the event-related
theta response depends on the development of the spontaneous theta activity
and how it varies with age in children. In the present study, a single-sweep
analysis was applied to assess the developmental changes in the event-related
EEG theta activity. Auditory passive, oddball target, and standard event-related
potentials (ERPs) were recorded at Fz, Cz, and Pz from 50 children aged
6-11 years and 10 young adults. Theta responses were analyzed in two time
windows of the post-stimulus epochs for three single-sweep parameters:
amplitude, phase-locking with stimulus, and enhancement relative to prestimulus
activity. For all three types of stimuli adults had theta responses with
lower amplitude, higher enhancement, and stronger phase-locking than those
of children. Unlike adults, no reliable differences between the early and
late theta response were found for children. Significant developmental
changes were observed for theta response amplitude that decreased and phase-locking
of early theta responses that increased with advancing age. These
findings indicate that the theta component of the auditory ERP differs
remarkably between children and adults and undergoes developmental alterations,
possibly reflecting specific differences in stimulus information processing.
Keywords: Event-related
potentials, theta response, phase-locking, children, EEG
Introduction
After external stimulation oscillatory electroencephalographic (EEG)
responses in different frequency ranges (theta, alpha, gamma, etc.) can
be recorded from the brain (Basar & Bullock, 1992). These oscillatory
EEG responses have been proposed to originate from the event-related reorganization
of the ongoing EEG as reflected by the synchronization, magnitude enhancement
or damping, and phase-reordering of the EEG after stimulation (Sayers,
Beagley, & Henshall, 1974; Parvin, Torres, & Johnson, 1980; Basar,
1980, 1992). Within this framework, the EEG frequency responses are strongly
associated with the background EEG activity. Given the natural changes
in the spontaneous EEG in the course of development (Niedermeyer, 1993),
as well as the functional relevance of event-related oscillations (Pantev,
Elbert, & Lütkenhöner, 1994; Basar, Hari, Lopes da Silva,
& Schürmann, 1997), the analysis of the EEG frequency responses
in children might provide a useful physiological approach to study developmental
brain functioning.
Event-related oscillations in the theta (4-7 Hz)
frequency range define the theta frequency component of the event-related
brain potentials (ERPs) or the EEG theta response. Prominent theta responses
have been observed in various experimental conditions in both humans and
animals and have been assigned an important role in integrative stimulus
processing (Miller, 1991; Schürmann & Basar, 1994). The
scalp recorded theta responses have been suggested to reflect the functioning
of a diffuse and distributed theta system in the brain (Basar-Eroglu, Basar,
Demiralp, & Schürmann, 1992) involving primarily the hippocampus
and associative frontal cortex (Miller, 1991; Basar-Eroglu et al., 1992;
Demiralp & Basar, 1992; Klimesch, Schimke, & Schwaiger, 1994) and
generating both the spontaneous and elicited theta oscillations (Basar,
1992).
The spontaneous EEG theta activity has been found
to decrease in absolute and relative band power with progressing age in
children (Matoušek & Petersén, 1973; John, Ahn, Prichep, Trepetin,
Brown, & Kaye, 1980; Matthis, Scheffner, Benninger, Lipinski, &
Stolzis, 1980; Gasser, Verleger, Bächer, & Sroka, 1988; etc.).
However, the information about theta response development is insufficient
although the event-related theta activity may also vary with age in children.
Firstly, the evoked potential magnitude is known to be significantly correlated
with the power of the background EEG but in many instances EEG and ERP
amplitudes have shown relatively independent behaviour (see e.g., Shagass,
1976). It is an open question whether a decrease in theta activity during
stimulus processing accompanies the developmental reduction of the spontaneous
EEG theta power. Secondly, increased theta activity in human adults has
been consistently associated with higher cognitive processes like memory,
concept learning, attention, etc. (Mizuki, Takii, Nishijima, & Inanaga,
1983; Lang, Lang, Diekmann, & Kornhuber, 1989; Basar-Eroglu et al.,
1992; Inouye, Shinosaki, Iyama, Matsumoto, & Toi, 1994; Klimesch et
al., 1994; Klimesch, Doppelmayr, Russeger, & Pachinger, 1996). Given
these correlations, it is striking that although the spontaneous EEG theta
power decreases with age in children and is relatively small in adults,
the efficiency of cognitive functioning improves in the course of development
(Piaget 1969). This implies that changes in theta system involvement during
event processing as reflected by the theta response should occur with
brain development. Finally, theta responses of young (three-year-old) children
were demonstrated to be larger, delayed, and more variable than those of
adults (Basar, 1982; Basar-Eroglu, Kolev, Ritter, Aksu, & Basar, 1994;
Kolev, Basar-Eroglu, Aksu, & Basar, 1994). Taken together, these findings
suggest that the EEG theta response undergoes specific developmental variations
but it is not known how and which response characteristics change as children
mature. Therefore, the present study aimed to assess age-dependent alterations
of EEG theta responses in 6-10-year-old children. Healthy young adults
were also studied to evaluate the mature theta response.
As mentioned above, the EEG frequency responses are
proposed to originate from the reorganization of the spontaneous EEG. Therefore,
theta response characteristics reflecting the stimulus-related changes
in the ongoing EEG should be regarded as relevant for analysis. Such characteristics
are the response phase-coupling to stimulus and amplitude enhancement or
suppression in the post-stimulus epoch (Sayers, Beagley, & Riha, 1979;
Kalcher & Pfurtscheller, 1995). In the averaged ERPs, however, amplitude
and phase-locking effects are confounded and cannot be analyzed separately
(see e.g., Ruchkin, 1988). To quantify theta response phase-locking independently
of amplitude effects, an original method for single-sweep analysis was
applied (Kolev & Daskalova, 1990; Yordanova & Kolev, 1996a; 1998;
Kolev & Yordanova, 1997). Thus, the major questions addressed in this
research were how single theta response amplitude, phase-locking, and enhancement
relative to prestimulus activity vary with age in children and whether
their developmental changes depend on the background EEG theta activity?
Previous studies on adults have shown that event-related
theta activity differs between processing conditions (Demiralp & Basar,
1992; Basar-Eroglu et al., 1992; Klimesch et al., 1994; Mecklinger, Kramer,
& Strayer, 1992; Yordanova & Kolev, 1998). To examine whether the
age-related variations in the theta response are restricted to a specific
processing condition, passive and task auditory stimuli were used. Since
the latency of the maximal theta response in the averaged ERPs has been
shown to be longer in younger children (Basar-Eroglu et al., 1994; Yordanova
& Kolev, 1996b), measurement and evaluation were made for early and
late post-stimulus epochs to enable assessment of single theta response
dynamics over time after stimulus appearance. The relationship between
single theta response parameters and the ongoing (prestimulus) theta band
power was also evaluated. Results of the alpha frequency range in the same
groups of children are presented in a related paper (Yordanova & Kolev,
1996a).
Methods
Subjects
A total of 50 healthy children from 6 to 10 years of age served as
subjects, with ten adults from 20 to 30 years of age also assessed. As
indicated in Table 1, the ages of children ranged
between 72 and 132 months, and were divided into 5 age groups consisting
of 10 subjects (4-6 females) each. Children were obtained from local schools
in Sofia and adult subjects were volunteers, primarily students from the
Medical University, Sofia. Children were selected from a large sample after
a neurological screening and were free of neurological disturbances. Interviews
with teachers and parents of the children revealed no signs of attentional,
behavioural, or learning problems. The children were of similar socio-economic
status (professional - executive) as assessed by parents' education and
occupation, and had normal and above IQ scores as indicated by Raven's
and verbal tests. Subjects were right-handed (Annett, 1985), denied
any history of neurologic, psychiatric, or hearing problems, and were paid
for their participation in the experiment.
Table 1. Distribution of the subjects studied
according to the age. Each group contains 10 subjects.
|
Children
|
|
|
|
|
Adults
|
|
6 years
|
7 years
|
8 years
|
9 years
|
10 years
|
20-30 years
|
| Age (years) |
6.50
|
7.60
|
8.50
|
9.30
|
10.60
|
24.10
|
| Age (months) |
77.90
|
91.20
|
102.10
|
111.50
|
127.10
|
289.20
|
| SD (months) |
4.65
|
3.25
|
4.68
|
3.03
|
4.43
|
44.40
|
Stimuli and procedure
The subjects were assessed in a dimly lit, electrically shielded room
and were monitored by means of a closed-loop TV and interphone system.
Children were given enough time before the recording sessions to become
acquainted with the environment.
The auditory stimuli were generated by a PC, filtered,
amplified, and reproduced by a loudspeaker in a free-sound field. All stimuli
were presented with intensity of 60 dB SPL and duration of 50 ms
(r/f 10 ms). The stimuli were delivered with random inter-stimulus intervals
(3.5-6.5 s) in two different task situations:
1) Tone bursts of 800 Hz frequency
(N = 50) were presented in a passive listening condition,
with subjects instructed to relax silently. Before the recording session,
they were told that the stimuli would be delivered for testing the technical
equipment and would be of no relevance for them.
2) Auditory target and nontarget stimuli
were presented in an oddball task. During
the task condition, 100 high and low frequency tones (1200 Hz and 800 Hz)
were delivered randomly, with probability P = 0.75 for the high
tones, and P = 0.25 for the low tones. Subjects were required to
press a button with their dominant hand as quickly and accurately as possible
in response to the low tones.
In both the passive and oddball conditions the subjects were keeping their
eyes closed.
Data collection and processing
Electrodes
The EEG was recorded with Ag-AgCl disc electrodes placed on midline
frontal, central and parietal sites (Fz, Cz, Pz), with linked mastoids
as a reference. The ground electrode was positioned on the forehead. The
electrooculogram (EOG) was recorded bipolarly with electrodes placed below
and at the outer canthus of the left eye. Electrode impedance did not exceed
10 kOhms.
EEG recording and data storage
EEG was amplified with cut-off frequencies of 0.5 and 70 Hz by means
of a Nihon Kohden Electroencephalograph (model EEG-4314F). Stop-band filtering
(band limits 48-52 Hz) was used for eliminating line frequency interference.
The amplified EEG analog signals were digitized with a sampling frequency
of 250 Hz (12 bit analog-to-digital converter) and stored for off-line
processing with epoch length of 1024 ms pre- and 1024 ms post-stimulus.
Reaction times (RTs) were recorded automatically.
Artifact rejection
The stored raw single sweeps were inspected visually off-line to eliminate
EEG segments contaminated with blink, muscular, or any other type of artifact
activity, with any EEG or EOG trial exceeding ±50 µV for adults
and ±90 µV for children excluded from further analysis. Thus,
the number of artifact-free sweeps analyzed for each subject in each condition
was between 40 and 50 for the passive and nontarget ERPs, and about 20-23
for the target ERPs.
Data analysis
Prestimulus EEG power spectral density
For each artefact-free single sweep, the power spectral density functions
were calculated for the prestimulus epochs (-1000, 0 ms) using the Fast
Fourier Transform (FFT), and then averaged separately for each stimulus
type. For statistical evaluation, the mean absolute band power (X) in the
range of 4-7 Hz, was log-transformed according to the formula Y = log10(X)
to normalize the power distributions (Gasser, Bächer, & Möcks,
1982; Gasser et al., 1988).
Digital filtering
Averaged and single-sweep ERPs were digitally band-pass filtered in
the theta frequency range (4-7 Hz). To provide a zero phase shift, a modified
linear band-pass filter was used, whose weights were based on binomial
coefficients (Wastell, 1979). The filter band width was adjusted to be
5% from the total analyzed frequency band, which was experimentally proven
to minimize filtering artifacts. Although in this study the main emphasis
is placed on single-sweep parameters, averaged unfiltered and filtered
(4-7 Hz) ERPs were also obtained to enable comparison with results from
the single-sweep analysis and with literature data.
Single sweep analysis
Three parameters of the single-sweep theta responses were analyzed
for two time windows, early (0-300 ms) and late (300-600 ms): (1)
maximal amplitude, (2) phase-locking, and (3) amplitude enhancement relative
to prestimulus theta activity.
1) The maximal peak-to-peak amplitude of the single theta responses
was measured in each of the two time windows. The mean value was calculated
for each subject, stimulus type, and electrode location.
2) For a quantitative evaluation of the phase-locking, a modification
of the single sweep wave identification (SSWI) method was applied (Kolev
& Daskalova, 1990; Kolev & Yordanova, 1997). The steps of the analysis
procedure are described below and are illustrated schematically in Figure
1.
First, extrema (minima and maxima) were identified in the filtered (4-7
Hz) single sweeps. The amplitudes of the identified extrema were coded
with +1 for the maxima and with -1 for the minima. Amplitude and latency
values of the identified extrema were stored. This step is illustrated
in Figure 1a where several representative single sweeps
are shown. Figure 1c illustrates the detected
points with coded amplitudes presented without the signals along the time
axis.
Second, a histogram of the number of phase-locked
single theta waves (single sweep wave identification histogram, SSWI histogram)
was constructed. To perform data reduction, the analysis epoch was divided
into time intervals of 20 ms. For each time interval the identified coded
extrema were summed across trials. Thus, the number of the phase-locked
waves in the consecutive single sweeps for each 20 ms interval was determined,
and the obtained value was assigned to the corresponding histogram bar
(Figure 1d). Typical SSWI histograms are shown also
in Figure 4c.
Third, quantitative evaluation of single-sweep phase-locking
was performed. The SSWI histogram was normalized by dividing the bar values
by the number of single sweeps included. The sum of absolute bar values
of the normalized SSWI histogram was calculated for the time windows 0-300
and 300-600 ms post-stimulus, thereby giving information about the strength
of single-sweep phase-locking in two consecutive post-stimulus periods.
The sums were computed for each subject, stimulus type, and electrode site.
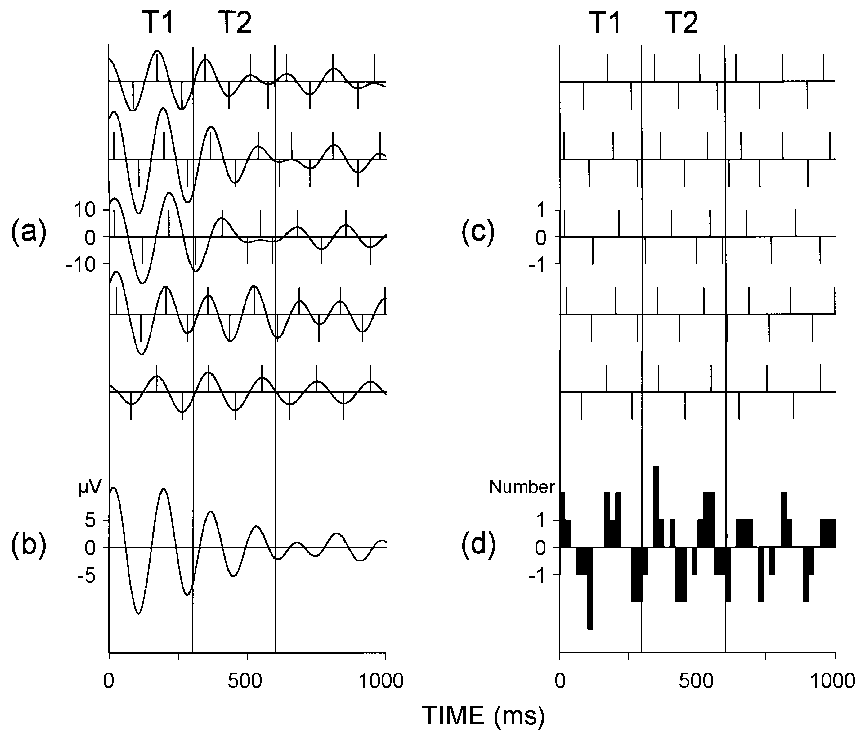
|
| Figure 1. Single Sweep Wave Identification
(SSWI) method. The left panel presents (a) five single sweeps filtered
in the theta range (4-7 Hz), and (b) their averaged waveform. The wave
extrema in the single sweeps are detected according to the local maxima
and minima, shown as vertical bars in (a). The same vertical bars (c) are
located at the corresponding latency positions without the signals, and
(d) the corresponding SSWI-histogram is build according to the rule:
in consecutive 20 ms time-lags +1 is added to the histogram bar if the
detected extremum in the single sweep is maximum, or respectively -1, if
the detected extremum is minimum. T1 - time window 0-300 ms, T2 - time
window 300-600 ms. |
3) Single-sweep enhancement relative to prestimulus activity
was analyzed by calculating the enhancement factor EF (Basar,
1982): For each single sweep N, the ratio of the maximal response
amplitude RN to the root mean square value rmsN
of the ongoing EEG amplitude prior the stimulus (in the time window -500,0
ms) was calculated according to the formula:
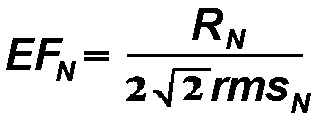 The term
The term 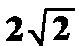 gives
the relation between the maximal amplitude and rms amplitude in case they
are equal. As tested experimentally, the post-stimulus amplitude is enhanced
if EF > 1.5 (Basar, 1982). EFs were calculated for the
early and late time windows. Thereafter, the mean values for each subject,
location, and stimulus were obtained.
gives
the relation between the maximal amplitude and rms amplitude in case they
are equal. As tested experimentally, the post-stimulus amplitude is enhanced
if EF > 1.5 (Basar, 1982). EFs were calculated for the
early and late time windows. Thereafter, the mean values for each subject,
location, and stimulus were obtained.
Statistical analysis
Each of the three parameters of single theta responses (individual
means of the maximal peak-to-peak theta amplitudes, individual means of
enhancement factors, and integral values of the normalized SSWI histograms)
was analyzed separately for the passive, target, and nontarget ERPs.
Individual values were subjected to repeated-measures analysis of variance
with one between-subject variable, AGE (6 levels corresponding to
each age group, 6, 7, 8, 9, 10 years, and adults), and two within-subjects
variables, TIME WINDOW (early vs. late), and ELECTRODE (Fz,
Cz, and Pz). The same analysis was performed for the maximal peak-to-peak
amplitudes of averaged filtered (4-7 Hz) ERPs. The Greenhouse-Geisser correction
was applied to the analyses with repeated measures factor ELECTRODE. The
original df and the probability values from the reduced df are reported
here. Results from testing simple effects not presented in tables, are
regarded as significant only if the probability values P were smaller
than 0.05. In order to describe group specific differences, post-hoc univariate
F-contrasts were performed. The Bonferroni procedure was used to correct
the probability values for the number of comparisons made. The corrected
probability values are reported in the results. A two-way ANOVA
(AGE x ELECTRODE) was performed on the log-transformed absolute theta-band
power values of the prestimulus EEG of the passive, target, and nontarget
ERPs, and one-way ANOVA (AGE) was performed for reaction time and
error rate data. To analyze the relationship between prestimulus theta
activity and theta response, step-wise multiple regression analyses were
carried out for the log-transformed absolute theta-band power and single-response
parameters. To evaluate the relationship between RT and event-related theta
activity, Pearson correlation coefficients were calculated for single theta
response parameters to targets at each lead and RTs.
Results
Behavioral data
As indicated by the significance of the age factor (AGE: F(5,54) =
14.5, P < 0.001), children's RTs to targets decreased with increasing
age (group means of 6-, 7-, 8-, 9-, and 10-year-old children: 716,
702, 675, 602, 472 ms), but were significantly slower than those of adults
(mean 390 ms). RTs did not differ between the groups of 6-8 year-olds,
who also manifested significantly slower responses than 9-10-year-old children.
Error rate was higher in children relative to adults, but this difference
was not significant.
Time domain averaged ERPs
Time domain ERPs are presented to illustrate that the present data
are consistent with previous results obtained with the classical ERP method.
Figure 2 shows that the ERPs of both children and adults
were characterized by N1, P2, N2, and P3 (P300) components. Children additionally
displayed a frontal late negative wave N400-700, and a parietal late positive
wave P400-700 that occurred primarily in response to the targets and decreased
in latency with advancing age in children. A detailed analysis of the time-domain
ERP components has been reported elsewhere (Yordanova, Angelov, Silyamova,
& Kolev, 1992).
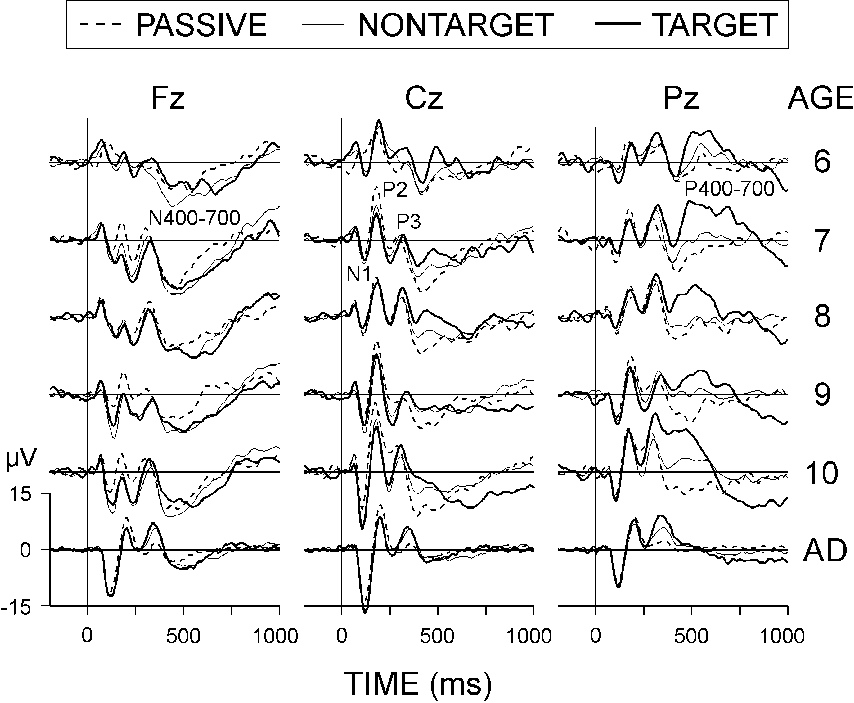
|
| Figure 2. Grand average passive, nontarget,
and target ERPs at three electrode locations (Fz, Cz, and Pz) from different
age groups: 6 year-olds, 7 year-olds, 8 year-olds, 9 year-olds, 10
year-olds, AD - adults. Each age group consists of 10 subjects. Stimulus
presentation is at 0 ms. |
Theta activity analysis
Prestimulus theta power
The values of the absolute theta power of the EEG preceding the passive,
target, and nontarget ERPs were first subjected to AGE x STIMULUS
x ELECTRODE analysis of variance. No significant main effect or interactions
of STIMULUS were obtained. The significant effect of AGE found for each
stimulus type resulted from the gradual decrease in theta power between
6 and 10 years, with the lowest values manifested by adults (AGE: F(5,54)
> 48.0, P < 0.001). The prestimulus theta power was highest
at Cz (ELECTRODE: F(2,108) > 7.8, P < 0.001).
Averaged filtered ERPs
Figure 3 illustrates that the maximal peak-to-peak
amplitude in the averaged (4-7 Hz) ERPs did not depend on the AGE (F(5,54)
< 0.64, P > 0.5). In 6-8-year-old children, the maximal theta
response at Pz and Cz occurred later (AGE X TIME WINDOW: F(5,54) > 3.37,
P < 0.05). It is also seen in the figure that the frontal theta
responses of children were delayed relative to those of adults. Maximal
values were found at Cz (ELECTRODE: F(2,108) > 6.1, P < 0.001).
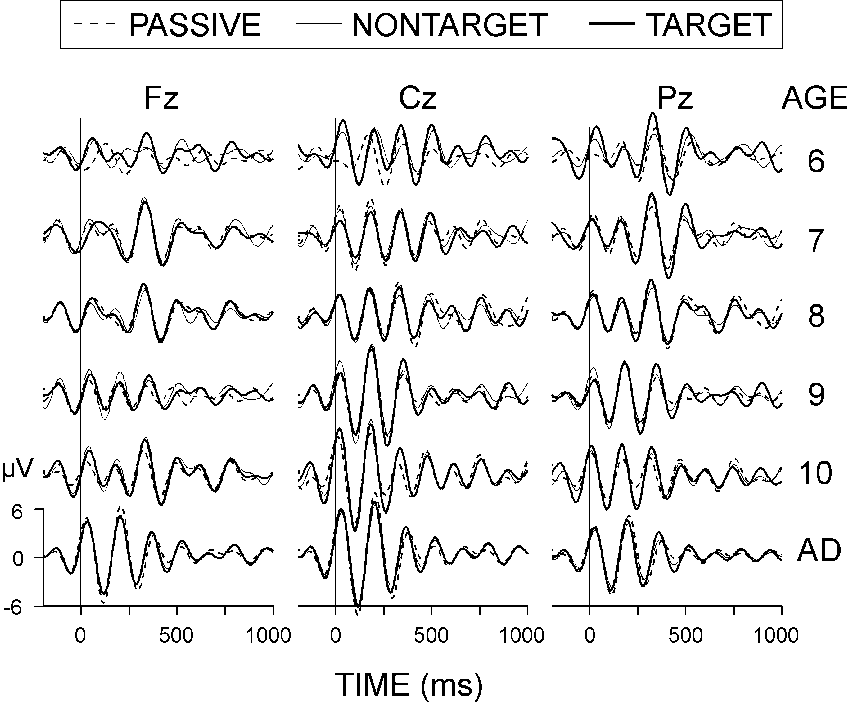
|
| Figure 3. Grand average passive, nontarget,
and target ERPs at three electrode locations (Fz, Cz, and Pz) filtered
in the theta (4-7 Hz) range. The age groups are designated as in Figure
2. |
Single-sweep analyses
Figure 4 displays representative individual data
to visualize the specific information reflected by each of the three analyzed
parameters. Results from statistical evaluation of single-sweep amplitude,
phase-locking, and enhancement factor are summarized in Table
2.
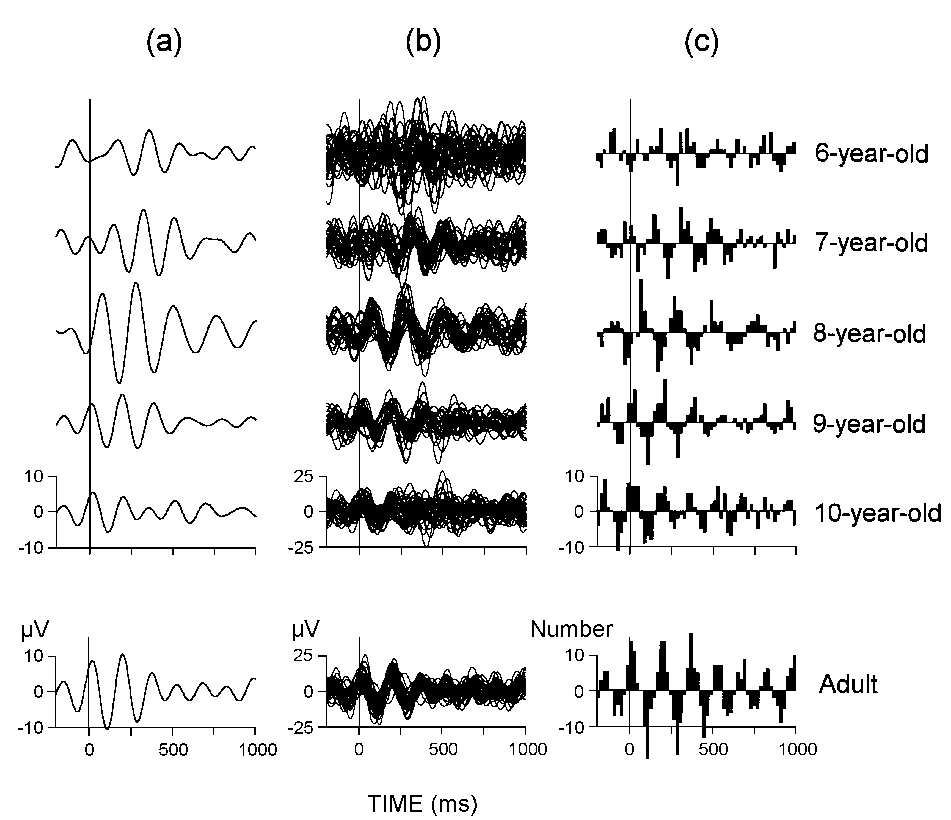
|
| Figure 4. (a) Averaged ERPs filtered in the
theta (4-7 Hz) range, (b) superimposed single sweeps filtered in the same
(4-7 Hz) range, and (c) the corresponding SSWI histograms for six representative
subjects at 6, 7, 8, 9, and 10 years of age, and an adult. Children had
larger single theta responses than adults, especially in the late time
window (300-600 ms). As reflected in the SSWI histograms, children also
displayed a weaker phase-locking in both the early and late time windows
than adults. All recordings are from the passive listening condition at
Cz. Along the Y-axes — amplitude (µV) for (a) and (b), and number
of synchronized single waves for (c). |
Table 2. Results (F values) from
statistical analysis of variance with repeated measures. Variables:
AGE (6-, 7-, 8-, 9-, 10-year-old children, adults), TIME WINDOW (0-300
ms, 300-600 ms), and ELECTRODE (Fz, Cz, Pz).
Parameters
| Source (df) |
| AGE (5,54) |
| TIME WINDOW (1,54) |
| A x T (5,54) |
| ELECTRODE (2,108) |
| E x A (10,108) |
| E x T (2,108) |
| E x A x T (10,108) |
|
Maximal peak-to peak
amplitude of single theta
response
| passive |
target |
nontarget |
| 12.3*** |
15.3*** |
17.9*** |
| 13.3*** |
6.3* |
9.2*** |
| 5.6*** |
3.2** |
4.5*** |
| 26.9*** |
20.6*** |
24.6*** |
| n.s. |
n.s. |
n.s. |
| 7.1*** |
4.3* |
15.1*** |
| n.s. |
n.s. |
n.s. |
*** P < 0.001, ** P < 0.01, |
Normalized Number of
phase-locked theta waves
| passive |
target |
nontarget |
| 21.3*** |
13.8*** |
14.4*** |
| 25.6*** |
15.8*** |
32.8*** |
| 8.6*** |
7.5*** |
10.2*** |
| 6.2*** |
6.9*** |
15.2*** |
| n.s. |
n.s. |
n.s. |
| 3.4* |
9.9*** |
15.7*** |
| n.s. |
n.s. |
2.1* |
* P < 0.05, n.s. not significant |
Enhancemet Factors
| passive |
target |
nontarget |
| 7.8*** |
10.4*** |
6.9*** |
| 17.3*** |
n.s. |
5.9* |
| 26.9*** |
7.4*** |
15.7*** |
| 6.0*** |
5.3** |
19.2*** |
| 2.0* |
n.s. |
n.s. |
| 7.2*** |
3.3* |
18.2*** |
| n.s. |
n.s. |
2.0* |
|
- Maximal peak-to-peak amplitude
As presented in Table 2, the statistical outcomes
were similar for the three types of stimuli and will be discussed in terms
of the overall patterns for the passive, target, and nontarget ERPs.
Figure 5a illustrates that:
(1) adults had significantly lower single-sweep amplitudes than children
from each group, with F(1/54) > 11.7 and P < 0.005 for each of
the univariate F-contrasts between single groups, (2) theta amplitudes
declined with age in children, and (3) the developmental time-courses differed
between the passive and task-related stimuli: For the target and
nontarget ERPs, no difference was found among the groups of 6-9 year olds,
and the ten-year-old children produced the lowest amplitudes compared to
the rest of children groups (F(1/54) > 6.94; P < 0.05 for each
contrast). For the passive ERPs, significant decreases were observed
for the groups of 7- and 9-year-olds (F(1/54) > 9.7; P < 0.05).
The significant main effect of TIME WINDOW was due
to the overall higher early than late theta responses. However, a significant
AGE x TIME WINDOW interaction also was found. Figure 5a
illustrates that only in adults and ten-year-old children (for targets)
were the early theta responses larger than the late ones. As indicated
by the significant ELECTRODE x TIME WINDOW interaction (Table
2), the difference between early and late theta responses was most
pronounced at Cz, with simple TIME WINDOW effect at Cz yielding P
< 0.001.
In sum, single theta response amplitudes decreased
with age in children and were lowest in adults. For each group, the early
theta responses were maximal at the vertex. The difference between early
and late response amplitudes was significant only in adults.
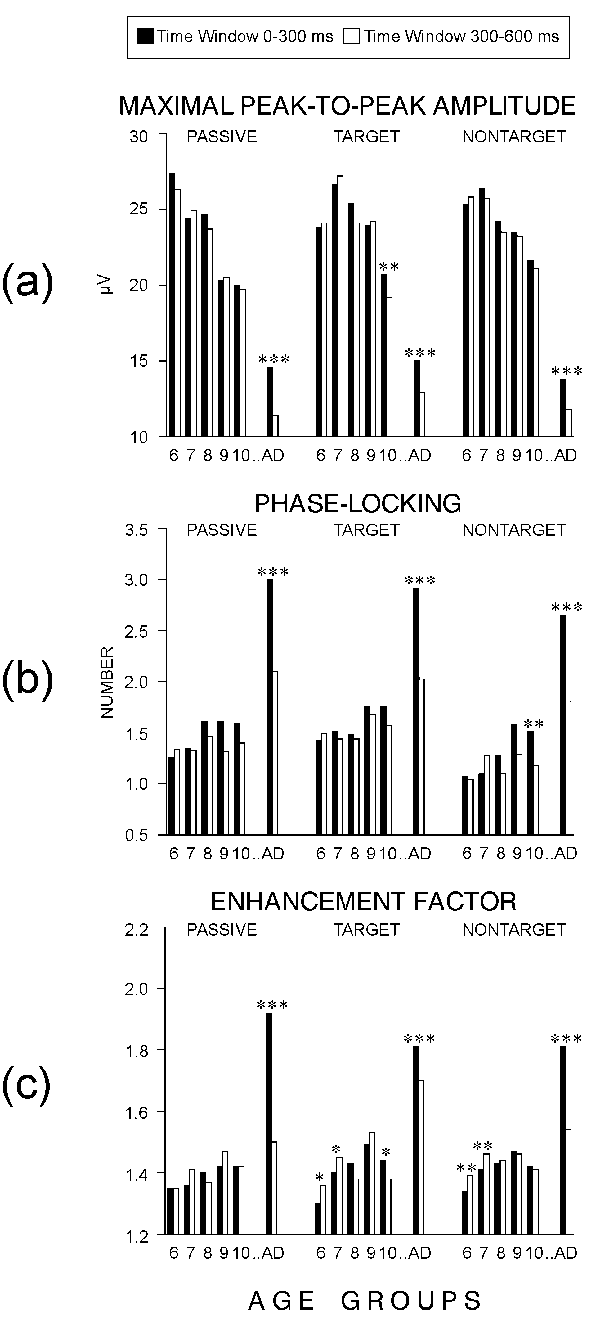
|
|
* P < 0.05, ** P < 0.01, *** P
< 0.001
|
| Figure 5. Effects of Time window on theta
responses to passive, target, and nontarget stimuli in six age groups:
(a) maximal peak-to-peak amplitude of the single theta responses, (b) number
of the phase-locked single theta waves, and (c) enhancement factor. The
age groups are designated as in Figure 2. |
- Phase-locking
The statistical results were again similar for the passive, target,
and nontarget ERPs (Table 2). Figure
5b demonstrates that the phase-locking was stronger in adults than
in children from each group (F(1/54) > 25.05; P < 0.005 for each
contrast). As indicated by the significant AGE x TIME WINDOW interaction
(P < 0.001), the phase-locking of the early theta responses increased
with age in children whereas no difference between children groups was
found for the late theta responses. Also, the early theta responses were
more strongly phase-coupled than the late ones (TIME WINDOW: P <
0.001) but the early vs. late difference was significant only for the adult
subjects (Figure 5b). As shown in Figure
6a, the developmental increase in the phase-locking of the early theta
responses was expressed mainly at Cz and much less evident at Fz and Pz.
These effects were statistically reliable only for the nontarget ERPs (AGE
x TIME WINDOW x ELECTRODE: P < 0.05). Also, the early but
not the late theta responses were best synchronized at the vertex (ELECTRODE:
P < 0.001; ELECTRODE x TIME WINDOW: P < 0.001).
In total, the phase-locking of theta responses was
significantly stronger in adults than in children. A developmental increase
was observed for the phase-locking of the early theta responses at the
vertex location.
- Enhancement factors
Figure 5c illustrates that the EFs of adults were
significantly larger than those of children at each age (AGE: P
< 0.001; and for each between-group contrast: F(1/54) > 32.3; P
< 0.005), with no statistical differences obtained among the groups
of children. The effect of the TIME WINDOW originated mainly from the significantly
greater early than late EFs in adults (AGE x TIME WINDOW: P <
0.001). Simple TIME WINDOW effects tested for each age group showed that
in contrast to adults, six-year-old children manifested significantly stronger
enhancement for the late than for the early theta responses in the task-related
ERPs, with no reliable early vs. late differences found for the rest of
the children groups, and for the passive ERPs (Figure 5c).
In addition, unlike adults, all children groups had greater enhancement
factors for the late than for the early theta responses to task-related
(target and nontarget) ERPs over the frontal brain area (AGE x TIME WINDOW
x ELECTRODE: P < 0.05 for the nontarget) - an effect illustrated
in Figure 6b. For adults the most pronounced differences
between early and late EFs were found at the mid-central location.
Altogether, the enhancement of theta responses relative
to prestimulus theta activity was substantially greater in adults than
in children. No increase in EFs was observed in children from 6 to 10 years.
In contrast to adults, children tended to produce a stronger enhancement
of the late theta responses, especially from 6 to 7 years of age, to task-related
stimuli and at the frontal site.
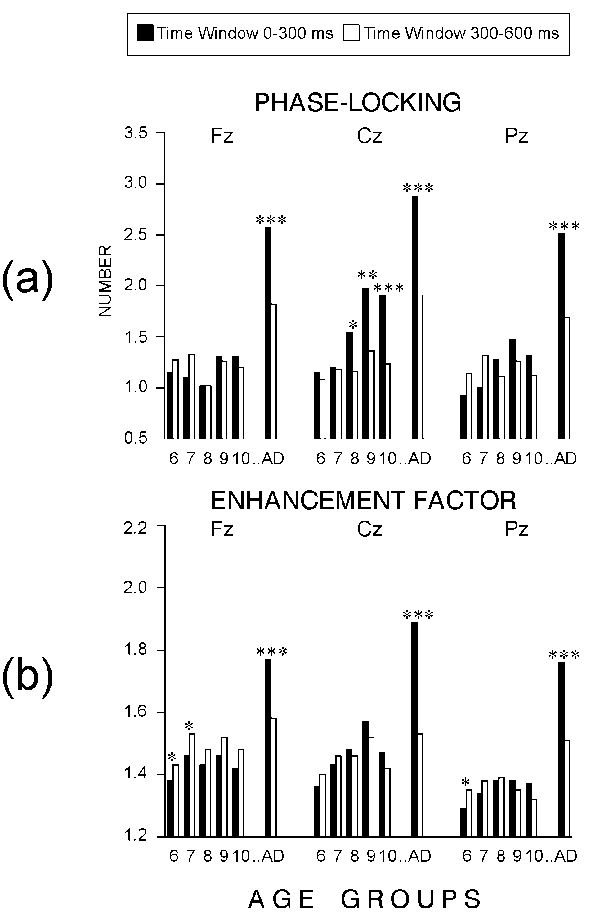
|
|
* P < 0.05, ** P < 0.01, *** P
< 0.001 |
| Figure 6. Effects of TIME WINDOW on theta
responses to nontarget stimuli from each ELECTRODE and AGE group:
(a) number of phase-locked single theta waves, (b) enhancement factor.
The age groups are designated in the same manner as in Figure
2. |
- Multiple regression analyses
The results obtained so far demonstrated the presence of developmental
variations in the amplitude and phase-locking of the early theta responses.
However, the prestimulus theta power also decreased with increasing age,
which may be responsible for the observed age-related changes in these
two single theta response parameters. To determine to what extent age influenced
theta response parameters due to the developmental power reduction of the
prestimulus theta activity, step-wise multiple regression analyses were
performed for children data from each stimulus type. The dependent variables
were single theta response amplitude and phase-locking, and the predictor
variables were log-transformed prestimulus theta power and children's age
in months. The results obtained were similar for the three stimulus types.
At each electrode site single theta response amplitude was entirely predicted
by the prestimulus theta power, since the AGE variable was removed from
the equations (R2total > 0.46, F(1/47) > 39.6, P <
0.001). In contrast, the stability of phase-locking at Cz depended only
on the age factor and was not predicted by variations of the prestimulus
theta power (R2total > 0.17, F(1/47) > 3.02, P < 0.005).
No significant correlations were found between children's reaction times
and single-sweep parameters of theta responses to oddball targets.
Discussion
The present study assessed the event-related theta activity in children
and adults at the level of single-sweep analysis. It was hypothesized that
the EEG theta responses would differ between children and adults as well
as among children groups, which can be evinced by differences in single-sweep
parameters. This hypothesis was confirmed and significant age-related variations
were found for single theta response amplitude, phase-locking, and enhancement
relative to prestimulus activity. Furthermore, these variations were specific
for each parameter. A major finding was that whereas single-sweep
amplitudes decreased, the phase-locking increased with age. As described
in the results, the averaged filtered ERPs did not manifest significant
differences between children and adults. It was because the smaller single-sweep
amplitudes of adults were accompanied by a pronounced phase-locking, and
the opposite was true for children. Thus, since additional information
was obtained that was obscured in the averaged waveforms, the developmental
changes in the EEG theta response could be revealed and evaluated precisely
at the level of single-sweep analysis.
Single-sweep theta responses in adults
In adults, the theta component of averaged auditory ERPs has been described
previously (Demiralp & Basar, 1992; Basar-Eroglu et al., 1992). In
the present study, by using single-sweep analysis the following new characteristics
of the event-related theta activity were found: (1) Auditory theta
responses display strong phase-locking and amplitude enhancement in the
post-stimulus epoch, which verifies the stimulus-related reorganization
of the ongoing theta activity in adults; (2) The early theta responses
(0-300 ms) are higher in magnitude and enhancement, and stronger in phase-locking
than the late ones (300-600 ms), with the differences between early and
late components being most pronounced at the mid-central location. These
observations were true for passive, target, and nontarget ERPs, which shows
that the early and late theta responses may reflect mechanisms activated
during both passive and task stimulus processing. Previous reports on adults
have found that enhanced (synchronized) event-related theta activity accompanies
short-term memory activation (Mecklinger et al., 1992; Klimesch et al.,
1994) and increased focused attention (Basar-Eroglu et al., 1992). These
memory- and attention-related effects were observed for the post-stimulus
epochs later than 250-300 ms. In addition, the late (300-600 ms) fronto-parietal
theta responses to target oddball tones have been reported to be more enhanced
and more strongly phase-locked than the late responses to passive stimuli,
whereas the early theta components did not differ between target and passive
stimulus processing (Yordanova & Kolev, 1998). Stimulus type effect
was not tested in the present study because only the developmental changes
in theta activity were focused on. However, the reports mentioned above
suggest that the early and late responses of adults as observed here may
relate to different processing mechanisms.
Differences in theta responses of children and adults
The organization of the auditory theta response was specific for children.
This was evidenced by the significant differences between the single-sweep
parameters of 6-10-year-old children and adults: (1) Single theta
responses of children were larger but not enhanced against prestimulus
theta activity, and also less synchronized than those of adults;
(2) In adults, the early theta oscillations expressed higher responsiveness
than the late ones, whereas in children the late responses were either
more enhanced than the early ones or no reliable differences were observed
between the early and late theta activity. It is noteworthy that the age-related
variations were similar for the three stimulus types. These findings generally
indicate that during auditory stimulus processing the theta response system
in adults operates in a manner different from that in 6-10-year-old children.
Since the passive and task-related stimuli produced similar age-related
differences, it may be further suggested that the specific organization
of the theta response in children reflects developmental variations of
basic stimulus-processing mechanisms common for the different processing
conditions. Such a proposal is supported by the observation that single
theta response parameters do not correlate with response speed.
Theta response system development
Single theta responses in children not only differed from those in
adults, but also changed with advancing age from 6 to 10 years: A
decrease in single-response amplitudes occurred at 10 years for the task
stimuli and at 7 and 9 years for the passive stimuli, with the values of
the mature theta response not achieved even by eldest (ten-year-old) children.
The developmental alterations in amplitude are not likely to result from
differences in cranial parameters such as thickness of the scalp or head
circumference (Polich, Ladish, & Burns, 1990; Gasser et al., 1988)
- a conclusion supported also by the different time courses of single theta
amplitudes for the passive and task stimuli. As revealed by the multiple
regression analysis results, these age-related amplitude effects resulted
exclusively from the developmental decrease in the power of the ongoing
EEG theta activity. Furthermore, the early theta responses displayed a
maximum at Cz as did the prestimulus theta power. The observation of the
strong relationship between the pre- and post-stimulus theta amplitudes
may be regarded as supporting the concept of the diffuse and distributed
theta system in the brain generating both the spontaneous and stimulus-related
4-7 Hz activity (Basar, 1992). Hence, the developmental reduction of EEG
theta activity may indicate a decrease in the number and/or intensity of
the neuronal elements that determine operative theta states of the brain.
The present results demonstrate, however, that single
theta responses of children are large but do not change relative to the
prestimulus theta activity after stimulation. Also, the ability to enhance
the magnitude of the evoked theta component does not improve from 6 to
10 years was not confirmed.
In parallel to the decrease in amplitude, an increase
in the phase-locking of early theta responses took place at about
9 years of age. However, for all three types of ERPs adults had remarkably
stronger phase-locking than any of the children groups. Hence, it may be
concluded that the capability to produce stable (congruent) theta responses
to auditory stimulation improves with development but appears related to
brain mechanisms that reach maturation at developmental stages later than
10 years.
The phase-locking in children did not depend on
the power of the prestimulus EEG theta activity. Instead, subject age was
the determinant of the developmental increase in phase-synchronization.
This result demonstrates that theta response phase-locking reflects processes
specifically activated after stimulus presentation. It is to be noted that
the developmental increase in phase-locking was found only for the early
theta responses and at the central site where the early theta responses
of adults were mostly expressed (Figure 6a). Although
the phase-locking of late responses was also significantly stronger in
adults than in children, no increase of late theta response phase-locking
was observed for children groups. These differential time courses support
the assumption that the early and late responses may have different functional
roles.
In addition, major developmental differences were
revealed for the time structure of all single-sweep parameters: Adults
manifested pronounced differences between the early and late theta responses,
but in children younger than 9-10 years the magnitude and phase-locking
of the early theta responses were similar to those of the late ones. Furthermore,
younger (6-7-year-old) children had a greater enhancement for the late
compared to the early theta responses (Figure 5c) and
all children groups tended to enhance the frontal late theta responses
more than the early ones (Figure 5b). Given the previous
results of adults outlined above, it cannot be ruled out that the relation
between early and late theta responses varies as a function of the specific
task conditions. Whether the reverse pattern in children reflects a response
delay, a different way of involvement of processes functionally specific
to the early and late responses in adults, or a qualitatively different
mode of organization of the event-related theta activity, is an open question.
The precise functional significance of the event-related theta activity
in both adults and children requires further investigation, especially
with respect to the meaning of single-sweep parameters. Nevertheless, the
present findings suggest that single theta response parameters and their
timing may provide a sensitive indicator of stimulus- and task-related
information processing in children.
Altogether, the single-sweep analysis results demonstrate
that the theta response system is not completely developed at the age of
10 years. At which age the adult values of single theta response parameters
are reached is not clear from the results but the processes related to
theta response system functioning obviously reach maturation at later stages
of development. It is to be emphasized that at the age of 10 years the
frontal lobes, unlike other brain structures, are reported not to have
reached functional maturity with respect to their anatomical structure
and input-output connections (Rothenberger, 1990). Miller (1991) has raised
the hypothesis that the EEG theta rhythm reflects the fronto-hippocampal
interplay during context processing. The present findings of the incomplete
development of theta responses at 10 years of age as well as of the delayed
enhancement of frontal theta responses in children support the notion about
the association between the theta response and frontal lobe processes.
In this regard, since frontal lobe functioning has been assigned a major
role for the occurrence and course of psychiatric disorders in children
(Rothenberger, 1990), event-related theta activity may appear informative
as a supplementary tool for such clinical studies (e.g., Rothenberger,
1995; Yordanova, Dumais-Huber, Rothenberger, & Woerner, 1997). It is
also to be noted that the developmental changes of single-sweep parameters,
although occurring at different ages, were rather step-wise than gradual,
which points to their possible relation with stages of cognitive development
(Piaget, 1969) but the precise correlations of single theta response parameters
with cognitive stage is still to be investigated.
Theoretical implications
The developmental time courses were different for theta response amplitude,
phase-locking, and enhancement. These differential developmental courses
might reflect sequential effects in the maturation of a common underlying
mechanism. Alternatively, they may point to that specific mechanisms might
be involved in the maturation of the processes related to each of the three
single-sweep parameters.
The strong phase-locking of theta responses in adults
shows that repeatable and stable waveforms are produced after stimulation.
In the framework of the concept of the theta system in the brain (Basar,
1992), the strong phase-locking is likely to result from stable (facilitated
or traced) connections between neuronal elements. Hence, it can be assumed
that the theta networks involved in responding during the early stage (0-300
ms) of stimulus information processing are stabilized or traced with development
in children so that repeatable patterns can be produced at the central
but not at the frontal and parietal locations. The increase in repeatability
of the early theta responses is accompanied by a decrease in magnitude,
which might reflect a developmental specialization of the theta networks
based on involving less but functionally defined elements (Courchesne 1990).
The large enhancement factors in adults suggest that the neuronal elements
responding in the theta channel can be coactivated (synchronized) simultaneously
by the external stimulus (Klimesch et al., 1994) but no developmental changes
were observed for the enhancement of the theta responses. With regard to
the three single-sweep parameters analyzed here, it might be assumed that
the theta response system changes with development in a way that enables
less but functionally specified elements to be coactivated simultaneously
under defined conditions. Reduction of active elements, selection of networks,
and capability of synchronizing them upon stimulation seem to follow differential
time courses.
Acknowledgements
Research was supported by the National
Scientific Research Fund at the Ministry of Education, Science and Technologies,
Sofia, Bulgaria, and the Deutsche Forschungsgemeinschaft,
Bonn, Germany (Contr. 436-BUL-113/76).
We thank Dr. A. Vankov and Dr.
T. Demiralp for software development, and V.
Silyamova for data processing. Special thanks are due to Dr.
John Polich for his support and most helpful comments.
References
Annett, M. (1985). Left, Right Hand and Brain: The Right Shift
Theory. Hillsdale, NJ: Lawrence Erlbaum, pp. 81-99.
Basar, E. (1980). EEG Brain Dynamics. Relation between EEG and Brain
Evoked Potentials. Amsterdam: Elsevier.
Basar, E. (1982). Theoretical problems in the evaluation of evoked potentials
in adults and children. In A. Rothenberger (Ed.) Event-Related Potentials
in Children. Basic Concepts and Clinical Application (pp. 35-54). Amsterdam:
Elsevier.
Basar, E. (1992). Brain natural frequencies are causal factors for resonances
and induced rhythms. In E. Basar & T. H. Bullock (Eds.) Induced Rhythms
in the Brain (pp. 425-467). Boston: Birkhäuser.
Basar, E., & Bullock, T. H., Eds. (1992). Induced Rhythms in the
Brain. Boston: Birkhäuser.
Basar, E., Hari, R., Lopes da Silva, F.H., & Schürmann, M.,
Eds. (1997). Brain Alpha-Activity - New Aspects and Functional Correlates,
International Journal of Psychophysiology, Vol. 26, Special issue.
Basar-Eroglu, C., Basar, E., Demiralp, T., & Schürmann, M.
(1992). P300-response: possible psychophysiological correlates in
delta and theta frequency channels. A review. International Journal of
Psychophysiology, 13, 161-179.
Basar-Eroglu, C., Kolev, V., Ritter, B., Aksu, F., & Basar, E. (1994).
EEG, auditory evoked potentials and evoked rhythmicities in three-year-old
children. International Journal of Neuroscience, 75, 239-255.
Courchesne, E. (1990). Chronology of postnatal human brain development:
Event-related potential, positron emission tomography, myelinogenesis,
and synaptogenesis studies. In J.W. Rohrbaugh, R. Parasuraman, & J.
Johnson, Jr. (Eds.) Event-Related Potentials: Basic Issues and Applications
(pp. 210-241). Oxford: Oxford University Press.
Demiralp, T. & Basar, E. (1992). Theta rhythmicities following
expected visual and auditory targets. International Journal of Psychophysiology,
13, 147-160.
Gasser, T. Bächer, P., & Möcks, J. (1982). Transformations
towards the normal distribution of broad band spectral parameters of the
EEG. Electroencephalography and Clinical Neurophysiology, 53, 119-124.
Gasser, T., Verleger, R., Bächer, P., & Sroka, L. (1988). Development
of EEG of school-age children and adolescents. I. Analysis of band power.
Electroencephalography and Clinical Neurophysiology, 69, 91-99.
Inouye, T., Shinosaki, K., Iyama, A., Matsumoto, Y., & Toi, S. (1994).
Moving potential field of frontal midline theta activity during a mental
task. Cognitive Brain Research, 2, 87-92.
John, E.R., Ahn, H., Prichep, L., Trepetin, M., Brown, D., & Kaye,
H. (1980). Developmental equations for the electroencephalogram. Science,
210, 1255-1258.
Kalcher, J. & Pfurtscheller, G. (1995). Discrimination between phase-locked
and non-phase-locked event-related EEG activity. Electroencephalography
and Clinical Neurophysiology, 94, 381–384.
Klimesch, W., Schimke, H., & Schwaiger, J. (1994). Episodic and
semantic memory: an analysis in the EEG theta and alpha band. Electroencephalography
and Clinical Neurophysiology, 91, 428-441.
Klimesch, W., Doppelmayr, M., Russeger, H., & Pachinger, Th. (1996).
Theta band power in the human scalp EEG and the encoding of new information.
NeuroReport, 7, 1235-1240.
Kolev, V. & Daskalova, M. (1990). Recognition and analysis of single
evoked potentials. In Proceedings of the North Sea Conference on Biomedical
Engineering. Antwerp.
Kolev, V., Basar-Eroglu, C., Aksu, F., & Basar, E. (1994). EEG rhythmicities
evoked by visual stimuli in three-year-old children. International Journal
of Neuroscience, 75, 257-270.
Kolev, V. & Yordanova, J. (1997). Analysis of phase-locking is informative
for studying event-related EEG activity. Biological Cybernetics, 76, 229-235.
Lang, M., Lang, W., Diekmann, V., & Kornhuber, H.H. (1989). The
frontal theta rhythm indicating motor and cognitive learning. In
R. Johnson, Jr., J. Rohrbaugh, & R. Parasuraman (Eds.) Current Trends
in Event-related Potential Research. Electroenceph. clin. Neurophysiol.
Suppl. 40. Amsterdam: Elsevier.
Matoušek, M. & Petersén, I. (1973). Frequency analysis of
the EEG in normal children and in normal adolescents. In P. Kellaway &
I. Petersén (Eds.) Automation of Clinical Electroencephalography
(pp. 75-102). New York: Raven Press.
Matthis, P., Scheffner, D., Benninger, Chr., Lipinski, Chr., & Stolzis,
L. (1980). Changes in the background activity of the electroencephalogram
according to age. Electroencephalography and Clinical Neurophysiology,
49, 626-635.
Mecklinger, A., Kramer, A., & Strayer, D. (1992). Event-related
potentials and EEG components in a semantic memory search task. Psychophysiology,
29, 104-119.
Miller, R. (1991). Cortico-Hippocampal Interplay and the Representation
of Contexts in the Brain. Berlin: Springer.
Mizuki, Y., Takii, O., Nishijima, H., & Inanaga, K. (1983). The
relationship between the appearance of frontal midline theta activity (Fmq)
and memory function. Electroencephalography and Clinical Neurophysiology,
56, 56-56.
Niedermeyer, E. (1993). Maturation of the EEG: Development of
waking and sleep patterns. In E. Niedermeyer & F. Lopes da Silva (Eds.)
Electroencephalography: Basic Principles, Clinical Applications and
Related Fields (pp. 167-191). Baltimore: Williams and Wilkins.
Pantev, C., Elbert, T., & Lütkenhöner, B., Eds. (1994).
Oscillatory Event-Related Brain Dynamics, NATO ASI Series, Vol. 271 (pp.
9-10). New York: Plenum Press.
Parvin, C., Torres, F. & Johnson, E. (1980). Synchronization of
single evoked response components: Estimation and interrelation of
reproducibility measures. In G. Pfurtscheller, P. Busser, F. Lopes da Silva,
& H. Petsche (Eds.) Rhythmic EEG Activities and Cortical Functioning
(pp. 203-217). Amsterdam: Elsevier.
Piaget, J. (1969). Psychology of intellect. In Selected psychological
studies (pp. 55-232). Moscow, in Russian.
Polich, J., Ladish, C., & Burns, T. (1990). Normal variation of
P300 in children: age, memory span, and head size. International
Journal of Psychophysiology, 9, 237-248.
Rothenberger, A. (1990). The role of frontal lobes in child psychiatric
disorders. In A. Rothenberger (Ed.) Brain and Behavior in Child Psychiatry
(pp. 34-58). Berlin: Springer.
Rothenberger, A. (1995). Electrical brain activity in children with
Hyperkinetic Syndrome: Evidence of a frontal cortical dysfunction.
In J. Sergeant (Ed.) Eunethydis. European Approaches to Hyperkinetic Disorder
(pp. 225-270). Zürich: Trümpi.
Ruchkin, D. (1988). Measurement of event-related potentials: Signal
extraction. In T. Picton (Ed.) Human event-related potentials, Handbook
of EEG, revised series, Vol. 3 (pp. 7–43). Amsterdam: Elsevier.
Sayers, B.McA., Beagley, H.A., & Henshall, W.R. (1974). The mechanism
of auditory evoked EEG responses. Nature, 247, 481-483.
Sayers, B.McA., Beagley, H.A., & Riha, J. (1979). Pattern analysis
of auditory evoked EEG potentials. Audiology, 18, 1–16.
Schürmann, M. & Basar, E. (1994). Topography of alpha and theta
oscillatory responses upon auditory and visual stimuli in humans. Biological
Cybernetics, 72, 161-174.
Shagass, Ch. (1976). Evoked potentials in man. In R.G. Grenell &
S. Gabay (Eds.) Biological Foundations of Psychiatry (pp. 199-253). New
York: Raven Press.
Wastell, D.G. (1979). The application of low-pass linear filters to
evoked potential data: filtering without phase distortion. Electroencephalography
and Clinical Neurophysiology, 46, 355–356.
Yordanova, J., Angelov, A., Silyamova, V., & Kolev, V. (1992).
Auditory event-related potentials in children under passive listening to
identical stimuli. Comptes rendus de l´Academie bulgare des Science,
45, 81-83.
Yordanova,
J. & Kolev, V. (1996a). Developmental changes in the alpha response
system. Electroencephalography and Clinical Neurophysiology, 99, 527-538.
Yordanova, J. & Kolev, V. (1996b). Brain theta response predicts
P300 latency in children. NeuroReport, 8, 277-280.
Yordanova, J., Dumais-Huber, C., Rothenberger, A., Woerner, W. (1997).
Frontocortical activity in children with comorbidity of tic disorder and
attention-deficit hyperactivity disorder. Biological Psychiatry, 41, 585-594.
Yordanova,
J. & Kolev, V. (1998). A single sweep analysis of the theta frequency
band during auditory oddball task. Psychophysiology, 35, 116-126.