Yordanova, J., Kolev, V. (1998). Event-related
alpha oscillations are functionally associated with P300 during information
processing. NeuroReport, 9: 3159-3164. Copyright © 1998
Lippincott Williams & Wilkins
Event-related alpha oscillations are functionally
associated with P300 during information processing
Juliana Yordanova* and Vasil
Kolev
Institute of Physiology,
Bulgarian Academy of Sciences, Acad. G. Bonchev str., bl. 23, 1113 Sofia,
Bulgaria
*Corresponding author:
Tel.: (359) 2-979-37-49
Fax: (359) 2-738-469
email: jyord@iph.bio.bas.bg
Abstract
Recent findings indicate that the electroencephalographic alpha (7-14
Hz) activity is functionally involved in cognitive brain functioning, but
the issue of whether and how event-related alpha oscillations may relate
to the processes indexed by the P300 component of the event-related brain
potentials (ERPs) has not been addressed. The present study assessed the
effect of auditory oddball task processing on slow (7-10 Hz) and fast (10-14
Hz) alpha activity from the P300 latency range. ERPs from mentally counted
targets (20%) and not counted nontargets (80 %) were recorded at Fz, Cz,
and Pz in nine subjects. Single-sweep phase-locking, power of phase-locked,
and power of non-phase-locked alpha responses during P300 activity were
quantified. The results demonstrated that larger and more synchronized
phase-locked fast alpha components at anterior (frontal-central) locations,
with reduced non-phase-locked slow alpha responses at the parietal site
were produced by targets relative to nontargets. Because the simultaneously
recorded P300 and alpha activity manifested a similar sensitivity to the
oddball task, event-related alpha appears to be functionally associated
with the cognitive processing demands eliciting P300. Also, evidence is
provided for the functional involvement of frontally synchronized and enhanced
alpha oscillations in task processing. NeuroReport 9: 3159-3164
© 1998 Lippincott Williams & Wilkins
Keywords: Cognitive alpha, Cognitive
processing, EEG, Event-related oscillations, Event-related potentials (ERPs),
P300, Phase-locking
Introduction
The processing of infrequently occurring target stimulus events activates
specific functional mechanisms reflected by the P300 (P3) component of
the event-related brain potential (ERP). Typically, larger P300 amplitudes
are obtained under task-relevant compared to passive processing conditions.[1]
Results from various experiments eliciting P300 have led to the suggestion
that P300 generation is associated with cognitive functioning such as memory
updating and attention allocation.[2,3]
Electroencephalographic (EEG) activity in the alpha (7-14 Hz) frequency
band also has been demonstrated to vary with cognitive brain processes.[4,5]
Event-related power changes of the EEG activity referred to as event-related
desynchronization (ERD) and synchronization (ERS) [5,6]
have revealed that conditions engaging attention and memory produce area-
and task-specific reduction of alpha activity within one second or more
after stimulation, such that the amount and duration of alpha power suppression
increase with increases in cognitive (memory) load, task relevance,
or surprise value.[5,7-9] Further,
mental task conditions requiring increased attention and intention [4,10,11]
or working memory activation [12] have been observed
also to elicit significant enhancements of EEG alpha waves. These findings
imply that EEG alpha frequency is functionally associated with the cognitive
activation or processes reflected by P300, but the precise nature of this
relationship is not known.[13]
In a previous report, P300 amplitude and alpha ERD were found to depend
in a similar manner on cognitive load and event rate, despite the distinct
latency epochs of P300 and ERD occurrence.[8] If ERD
amplitude and P300 are indeed different cortical indices of the same processes
[7], it may be expected that when P300 emerges as an
objective and time-localized marker of specific cognitive activation, EEG
alpha activity within the same time period would also vary with task demands.
Therefore, the objective of the present work was to assess the effects
of auditory oddball task processing on the event-related alpha activity
from the P300 latency range.
EEG oscillations following external stimulation may be tightly or loosely
phase-coupled with stimulus.[14] During auditory P300
elicitation, alpha activity has been described to desynchronize [15],
but prolonged phase-locked alpha activity also has been reported in auditory
tasks.[15,16] In this regard, to analyze precisely
P300-related alpha activity, the power of both the phase-locked and non-phase-locked
alpha responses was measured in the present study.[17]
In addition, to assess whether oddball task processing may affect the stability
and repeatability of alpha patterns, the phase-locking to stimulus of single-sweep
alpha responses within P300 was quantified independently of amplitude effects.[18-20]
Materials and Methods
Nine healthy 18-30 year-old adults (5 females) were assessed. None
reported any neurologic, psychiatric disorders, or hearing problems. Auditory
stimuli were presented in an oddball condition with intensity of 60 dB
SPL, duration of 1000 ms (r/f 10 ms), interstimulus intervals between 3.5
and 6.5 s. Two tone bursts of 2000 and 1950 Hz (N = 100) were delivered
randomly, with P = 0.2 for the lower (target) tones. Subjects kept
their eyes closed and counted mentally the rare targets. The EEG data were
recorded at Fz, Cz, and Pz with linked-mastoids as a reference using a
0.1-70 Hz band pass, with a sampling frequency of 250 Hz /12 bit. EEG segments
contaminated with ocular or muscular activity, or exceeding ±50
µV, were excluded from further analysis. For each subject, 16-18
artifact-free target sweeps were processed. The same number of artifact-free
sweeps was chosen randomly from the nontarget ERPs.
Averaged target and nontarget ERPs were obtained to measure the time-domain
P300 component. Event-related alpha activity was evaluated in two frequency
ranges: 7-10 and 10-14 Hz. Three parameters were measured:
(1) power of phase-locked activity, (2) power of non-phase-locked activity,
and (3) single-sweep phase-locking. Digital filtering was performed by
using a modified linear pass band filter providing zero phase shift, with
filter weights based on binomial coefficients. The filter band width was
greater than 5% from the total analyzed frequency band, which was experimentally
tested to minimize filtering artifacts. To achieve this ratio, the original
signals were downsampled to 125/s, which introduced no distortion in the
signal. The exact half-power frequencies of the digital filters were 6.84,
10.25, and 14.16 Hz, referred to as 7, 10, and 14 Hz in the text. The length
of the filtered single-sweep epochs was 2048 ms (-1024, +1024 ms), so that
possible edge effects did not alter the analyzed epoch.
To obtain phase-locked alpha power, single sweeps were filtered,
then averaged and squared. Non-phase-locked alpha power was calculated
according to the intertrial variance method [17] based
on the following procedure: From each single sweep band pass filtered
in the alpha range, the averaged ERP filtered in the same range was subtracted.
The resulting sweeps were then squared and averaged, thus obtaining the
instantaneous non-phase-locked power. Only for the sake of presenting alpha
reactivity in a manner comparable with other studies, ERS/ERD curves were
obtained by means of the Hilbert transform.[21] For
quantitative evaluation of single-sweep phase-locking, the single-sweep
wave identification (SSWI) method was applied.[18-20]
A histogram of the number of phase-locked single-sweep alpha waves (SSWI
histogram) was obtained according to the following procedure: The
filtered single sweeps were coded such that the extrema were replaced with
(+1) or (-1) for maxima and minima, respectively. The time points not belonging
to the extrema were replaced by zero. Thus, for each sampling point, the
sum of the identified coded (+1, -1) extrema was calculated for the trial
set and the number of the phase-locked waves was determined. The obtained
value was represented in a corresponding histogram bar. The histogram was
normalized according to the number of single sweeps used for analysis.
For statistical evaluation, mean power values of phase-locked
and non-phase-locked activity in the two frequency ranges were measured
for the time window 250-600 ms, in which P300 was expressed (Fig.
1). Power values were log10-transformed to normalize the distribution.
The sum of the absolute histogram values was calculated for the same time
window (250–600 ms). Measurements of slow and fast alpha activity were
made for each subject, stimulus type, and electrode site. Each parameter
was subjected to a repeated-measures analysis of variance with two within-subjects
variables: stimulus (target vs nontarget), and electrode (Fz,
Cz, Pz). P300 latency was measured as the latency of the maximum ERP peak
within 250-600 ms from stimulus onset. P300 amplitude was measured relative
to a prestimulus baseline of 200 ms before stimulus. P300 amplitude and
latency values were subjected to 2 stimulus x 3 electrode analyses of variance.
The Greenhouse-Geisser correction was applied to the analyses with repeated
measures factor electrode. The original df and corrected probability
values are reported throughout the text.
Results
P300: Figure 1 illustrates that
P300 amplitude was significantly larger (F(1/8) = 34.08, P <
0.001) and P300 latency was significantly longer (F(1/8) = 6.2, P
< 0.05) to targets than to nontargets. P300 amplitude demonstrated a
parietal maximum (F(2/16) = 22.3, P < 0.001).
Phase-locked alpha activity: Figure
1 (left panel) displays the time course of phase-locked alpha activity.
For 7-10 Hz components, a prominent power increase with a maximum at around
150-200 ms was observed for both stimulus types. For 10-14 Hz activity,
a biphasic power increase was detected at anterior locations within 1 s
after stimulus on-set. The second enhancement of phase-locked 10-14 Hz
responses occurred substantially later for the nontargets. Figure
2 illustrates the effects of stimulus type and electrode on phase-locked
alpha activity from the P300 range. The power of phase-locked 7-10 Hz responses
did not depend on stimulus and electrode. In contrast, phase-locked 10-14
Hz activity was most pronounced at frontal and central locations (F(2/16)
= 4.55, P < 0.05), and was significantly larger for targets than
for nontargets (F(1/8) = 6.3, P < 0.05) - effects seen also in
Fig. 1.
Non-phase-locked alpha activity: Figure
1 (middle panel) presents time courses of non-phase-locked alpha activity
and shows that at posterior sites (Pz and Cz) targets produced prominent
alpha power decreases reaching maximum at 500-1000 ms. As illustrated in
Fig. 2, the slow alpha (7-10 Hz) variance within
P300 latency range was significantly smaller for targets at Pz (stimulus
x electrode, F (2/16) = 4.8, P < 0.05). No statistically significant
effects were found for the non-phase-locked 10-14 Hz activity.
Alpha ERS/ERD: Figure 1 (right
panel) illustrates that P300 time window coincided with the transition
of ERS to ERD. As argued previously [15,17],
ERS corresponded to the enhancement of phase-locked and non-phase-locked
responses, while ERD dynamics was underlined by the non-phase-locked responses.
Although not quantified in the present study, it can be seen in the figure
that at Fz, the ERS of 10-14 Hz activity within P300 was much larger for
the targets.
Single-sweep alpha phase-locking: As depicted in
Fig. 2, the phase-locking of 7-10 Hz responses within
P300 range did not depend on stimulus type and electrode, while the phase-locking
of 10-14 Hz responses was significantly stronger for the targets (F(1/8)
= 8.80, P < 0.05) and at anterior than at parietal locations
(F(2/16) = 3.92, P < 0.05).
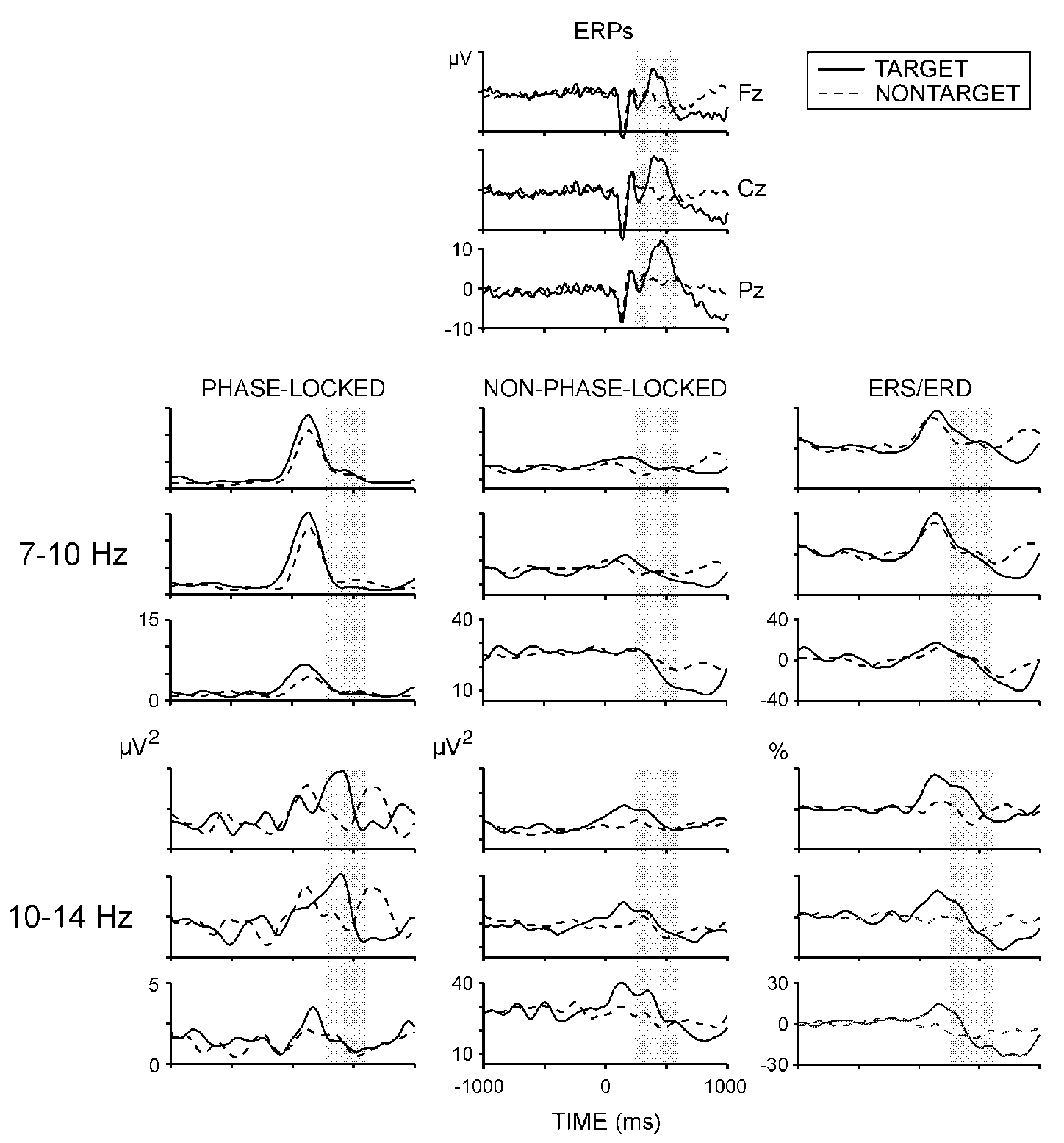
|
| Fig. 1. Grand average ERPs, phase-locked and non-phase-locked alpha
power, and ERS/ERD at three electrode locations. Shaded areas designate
the time window used for analysis. Stimulus starts at 0 ms and lasts till
1000 ms. |
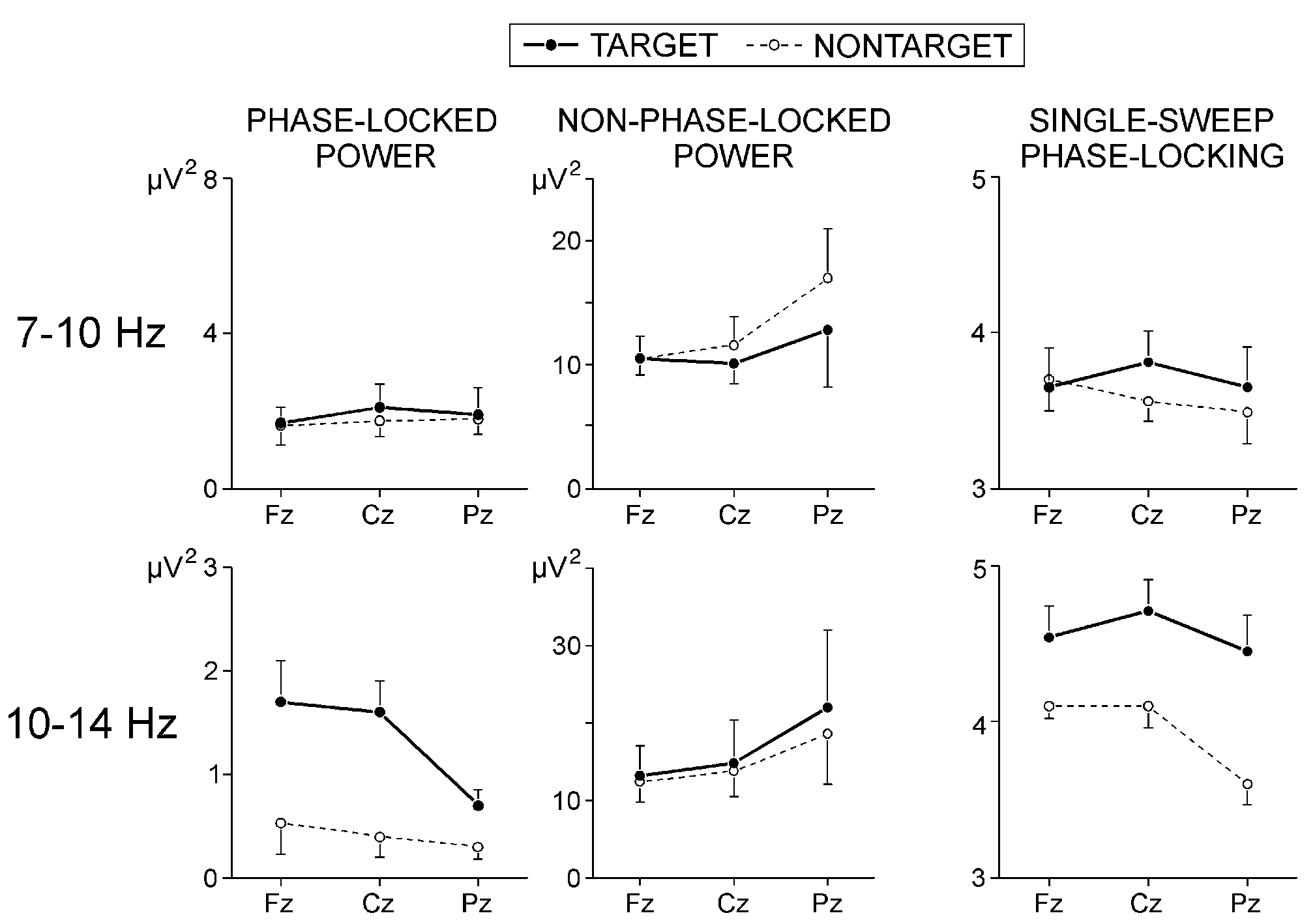
|
Fig. 2. Effects of stimulus type and electrode on the phase-locked
and non-phase-locked alpha power, and on the normalized number of phase-locked
single alpha waves in the time window 250-600 ms.
Values are mean +(-) s.e. |
Discussion
The functional role of EEG alpha activity in cognitive brain functioning
has been emphasized in previous reports [4-9,12],
but the issue of whether and how event-related alpha oscillations may reflect
the processes activated during and indexed by the endogenous P300 ERP component
has not been considered. The present study separately quantified the power
of phase-locked, the power of non-phase-locked, and the phase-locking of
alpha responses coinciding with P300. The results demonstrated that both
the phase-locked and non-phase-locked alpha oscillations during auditory
P300 were functionally relevant to the oddball task processing. These
findings provide new evidence for (1) the association of event-related
alpha activity with the processes eliciting P300, (2) the functional involvement
of synchronized and enhanced frontal alpha oscillations in task processing,
and (3) a putative mechanism that may account for the more synchronized
and ordered alpha states that accompany cognitive processes activated for
target stimulus processing and P300 generation.
Event-related alpha activity and P300: Previous
reports have shown that enhanced and phase-locked alpha oscillations can
be consistently observed shortly after auditory and visual stimuli, e.g.,
in the first 250 ms [4,9,14,19].
These synchronized alpha oscillations, called the alpha response [14],
typically coincide with the exogenous ERP components.
A major finding of the present study was that the power and pattern
stability of phase-locked 10-14 Hz activity within the P300 range (250-600
ms) were significantly larger for the mental count targets than for nontargets.
It is noteworthy that phase-locked alpha components were obtained for a
latency period much later than that of the classical alpha response, as
noted previously for another auditory task condition.[16]
It is not likely that spectral P300 components are responsible for the
observed task effects: First, in line with many previous reports
(rev. Ref. 1), P300 was with a parietal maximum whereas
the synchronization and power of phase-locked 10-14 Hz activity were maximal
at frontal and central locations (Figs. 1
and 2). Second, the dominant spectral components of P300 have been
recognized to belong to sub-delta, delta, and theta frequency ranges [15,22,23],
which is also implied by the present ERP results (Fig.
1). Thus, although fast alpha (10-14 Hz) energy may participate in
the frontal-central portions of P300, the larger and better synchronized
10-14 Hz oscillations appear to reflect a frontally distributed specific
state or process activated during the mental count condition. However,
the synchronized fast alpha oscillations occurred simultaneously with P300
and, like P300, were functionally relevant to the oddball task processing.
Hence, it may be concluded that the process reflected by fast alpha synchronization
at frontal locations is functionally associated with the major cognitive
demands eliciting P300, e.g., attention allocation and working memory activation.[2,3]
This conclusion is supported by previous findings according to which a
substantial increase of fast (10-12 Hz) alpha activity is produced over
large frontal and central regions by auditory stimulus memorization and
retrieval, and by visual attention [12]. In these
experiments, fast alpha ERS was maximal at 250-500 ms after stimulation
and lasted until 500-700 ms during attention and retrieval from memory,
and much longer during memorization.
Furthermore, increased attentional demands in auditory oddball tasks
have been recently reported to produce significant increases in fast alpha
power of the ERP epoch.[22] The power- independent
augmentation of phase-locking to targets as observed here (Fig.
2) additionally indicates that fast alpha synchronization within P300
is associated with stimulus-specific processing rather than with processing
of task in general. These findings strongly emphasize the role of enhanced
and synchronized alpha oscillations in higher brain functioning [10,4,11].
Concurrently, the parietal non-phase-locked slow alpha (7-10 Hz) activity
within P300 was more suppressed to targets than to nontargets, which resulted
in ERD expression (Fig. 1, Ref. 15).
This observation is consistent with previous ERD reports demonstrating
that alpha power is reduced by relevant stimulation, such that alpha attenuation
is maximal at posterior sites and increases with cognitive load and stimulus
significance [5,7-9,12].
The alpha reduction reported here, though differentiating targets from
nontargets during P300, reached maximum much later than P300 peaking (Fig.
1, see also Refs. 7,8). Hence, the mechanisms reflected
by the parietal slow alpha suppression may appear secondary to P300. As
slow alpha ERD has been related with unspecific attentional processes [5,7],
such processes may be triggered by the stimulus evaluation performed during
P300.[3] Because no task effects were detected for
the non-phase-locked fast alpha, it may be further proposed that the memory
processes supposed to be associated with the ERD of fast alpha activity
[5] are not identical to those eliciting P300.
According to the present results, during P300 time window, the frontal
increase in phase-locked fast alpha activity to targets was accompanied
by a parietal suppression of non-phase-locked slow alpha activity. Following
the classical interpretation, the simultaneous existence of ERS and ERD
in distinct scalp areas is explained by accepting that ERD reflects functionally
activated cortical regions, and ERS manifests a temporary inactivity in
other cortical fields.[6,5] However,
it seems improbable that frontal areas are functionally inactive during
the cognitive demands imposed by the mental count task, with targets producing
even greater frontal inactivity than nontargets, as may be concluded if
phase-locked alpha power increase would manifest a cortical state of rest
[24] (for a similar result see Refs. 12,
22). Another possible interpretation of the present observations is
that there are anterior and posterior alpha systems in the brain that are
as functionally separated as to employ entirely different frequencies (slow
and fast) and mechanisms (synchronizing and desynchronizing) during relevant
event processing. Although frontal and occipital generators of alpha activity
have been proposed to exist [25], the scalp topography
of slow and fast alpha ERD reported so far has demonstrated no clear frontal
vs. posterior differences between the two frequency bands. Instead, slow
alpha ERD was reported to be widely distributed over the scalp, while fast
alpha ERD was found to be more localized.[5,9,12]
Alternatively, within the concept of diffuse and selectively distributed
alpha systems in brain [14,4],
the topography-specific coexistence of suppressed non-phase-locked slow
alpha activity and enhanced phase-locked fast alpha activity may be regarded
as a higher-order alpha state, during which the non-phase-locked (or disordered)
alpha is minimized and the phase-locked (or ordered) alpha is maximized.
Such a viewpoint permits to regard the well known ERD phenomenon not merely
as a reduction of alpha power, but as a mechanism which, in addition to
phase-ordering, tends to stabilize alpha brain states in relation to relevant
information processing.
Conclusion
The present findings clearly demonstrate that event-related alpha activity
is associated with the processes eliciting auditory P300 ERP component,
such that, during P300 generation, frontal alpha oscillations are increased
and synchronized, and parietal alpha activity is suppressed to targets
than to nontargets. Thus, new evidence is provided for the functional involvement
of synchronized and enhanced frontal alpha oscillations in task processing.
ACKNOWLEDGEMENTS: Research was supported
by the National Research Fund by the Ministry of Education and Science,
Sofia, Bulgaria (Project B-703/97). Thanks are due to Dr. John Polich for
comments and suggestions.
References
[1] Polich J. J Clin Neurophysiol 15, 14-33
(1998).
[2] Donchin E and Coles MGH. Behav Brain Sci
11, 357-374 (1988).
[3] Polich J and Kok A. Biol Psychology 41,
103-146 (1995).
[4] Basar E, Schürmann M, Basar-Eroglu C et
al. Int J Psychophysiol 26, 5-30 (1997).
[5] Klimesch W. Int J Psychophysiol 26, 319-340
(1997).
[6] Pfurtscheller G and Klimesch W. Event-related
synchronization and desynchronization of alpha and beta waves in a cognitive
task. In: Basar E and Bullock TH, eds. Induced Rhythms in the
Brain. Boston: Birkhäuser, 1992: 117-128.
[7] Boiten F, Sergeant J and Geuze R. Electroenceph
Clin Neurophysiol 82, 302-309 (1992).
[8] Sergeant J, Geuze R and van Winsum W. Psychophysiology
24, 272-277 (1987).
[9] Sterman MB, Kaiser DA and Veigel B. Brain
Topogr 9, 21-30 (1996).
[10] Ray W and Cole H. Science 228, 750-752
(1985).
[11] Shaw J. Int J Psychophysiol 24,
7-23 (1996).
[12] Krause C, Lang H, Laine M et al. Brain
Topogr 8, 47-56 (1995).
[13] Polich J. Electroenceph Clin Neurophysiol
104, 244-256 (1997).
[14] Basar E. EEG Brain Dynamics: Relation
between EEG and Brain Evoked Potentials. Amsterdam: Elsevier,
1980.
[15] Van Dijk J, Caekebeke J, Jennenkens-Schinkel
A et al. Electroenceph Clin Neurophysiol 83, 44-51 (1992).
[16] Kolev V and Schürmann M. Int J Neurosci
67, 199-213 (1992).
[17] Kalcher J and Pfurtscheller G. Electroenceph
Clin Neurophysiol 94, 381-384 (1995).
[18] Kolev V and Yordanova J. Biol Cybern
76, 229-235 (1997).
[19] Yordanova
J and Kolev V. Electroenceph Clin Neurophysiol 99, 527-538 (1996).
[20] Yordanova
J and Kolev V. Psychophysiology 35, 116-126 (1998).
[21] Clochon P, Fontbonne J-M, Lebrun N et al.
Electroenceph Clin Neurophysiol 98, 126-129 (1996).
[22] Kolev
V, Demiralp T, Yordanova J et al. NeuroReport 8, 2061-2065 (1997).
[23] Spencer K and Polich J. Psychophysiology
(in press).
[24] Fuster J. The Prefrontal Cortex. Anatomy,
Physiology, and Neuropsychology of the Frontal Lobe. New York:
Raven Press, 1989.
[25] Inouye T, Shinosaki K, Yagasaki A et al. Electroenceph
Clin Neurophysiol 63, 353-360 (1986).
Received 1 July 1998;
accepted 23 July 1998


